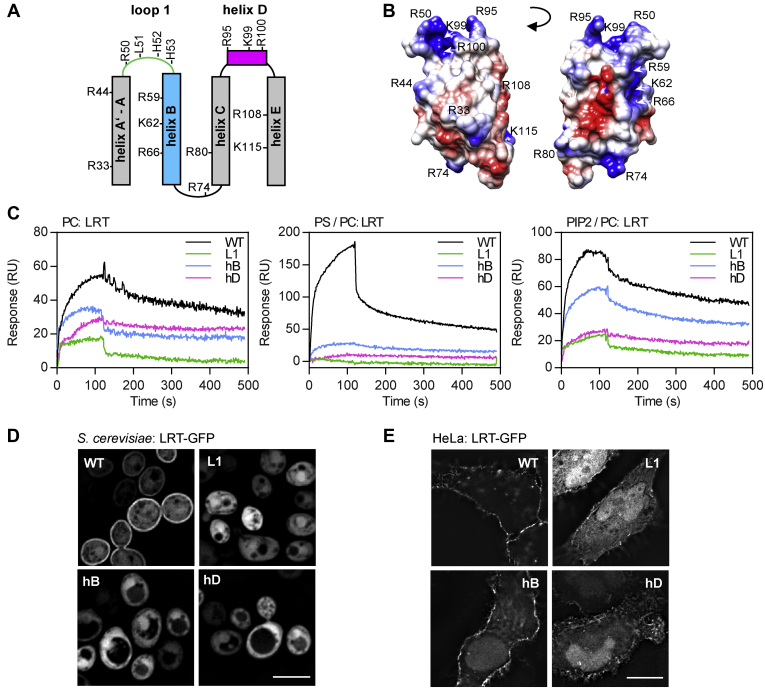
Figure 4.
Electrostatic interactions determine the membrane interaction of the LRT domain.A and B, topology diagram (A) and coulombic surface coloring (B) of the LRT helix bundle fold (aa 29–121, PDB code: 6RGN) with the indication of positively charged arginine and lysine residues. The color gradient of coulombic surface coloring, as calculated by Chimera 1.14rc, ranges from the positive charge in blue to the negative charge in red. C, overlay plot of SPR sensograms of the interaction between LRT protein variants and lipid membranes. Wildtype LRT (WT) and its charge-reversal substitution L1 (R50E + H52E + H53E), hB (R59E + K62E + R66E), and hD (K99E + R100E) variants were injected at 250 nM concentration over the neutravidin sensor chip coated with the immobilized lipid vesicles containing PC, PS/PC (20:80), or PIP2/PC (5:95). The sensograms show the representative binding curves obtained from six independent “one-shot kinetic” experiments. D and E, localization of the GFP-tagged LRT protein variants. D, S. cerevisiae BY4741 cells harboring plasmids encoding the indicated LRT–GFP protein variants were induced for 20 h for protein expression and examined by live-cell imaging. Scale bar, 5 μm. E, HeLa cells were transiently transfected with plasmids expressing the indicated GFP-tagged LRT proteins, fixed after 18 h, and examined by fluorescence microscopy. Scale bar, 20 μm. Representative images from two independent experiments with the same outcome are presented. hB, helix B; hD, helix D; L1, loop1; LRT, lipid raft targeting; PC, phosphatidylcholine; PIP2, phosphatidylinositol 4,5-bisphosphate; PS, phosphatidylserine; SPR, surface plasmon resonance.