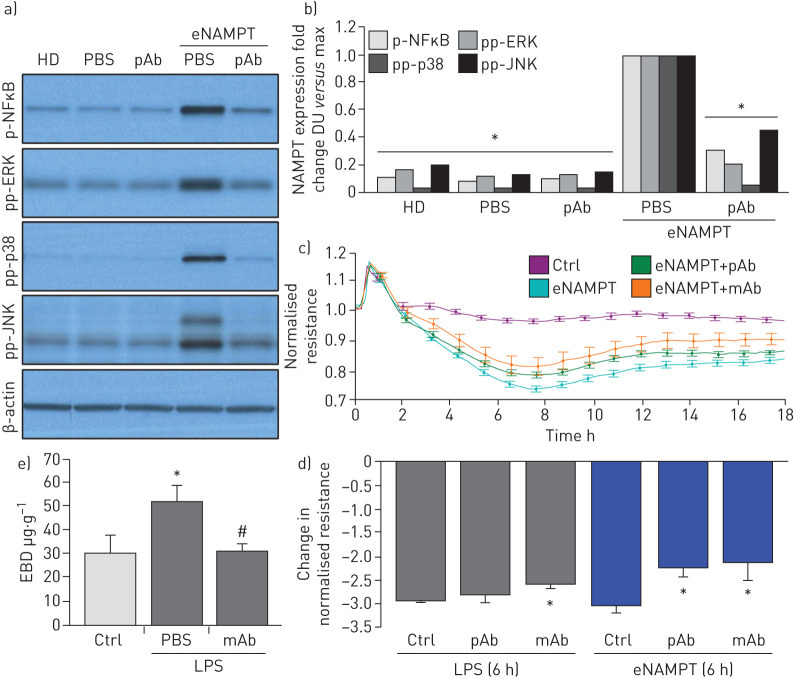
FIGURE 5.
Extracellular nicotinamide phosphoribosyltransferase (eNAMPT)-neutralising strategies attenuate eNAMPT-induced human lung endothelial cell (EC) signalling and barrier responses. a),b) Human lung ECs were challenged with either human recombinant eNAMPT alone (1 μg·mL−1, 1 h) or an eNAMPT-polyclonal antibody (pAb) mixture (eNAMPT 1 μg·mL−1+pAb 10 μg·mL−1, 1 h). Cells were next lysed and probed for phospho-proteins and total β-actin via Western blot. eNAMPT-induced robust phosphorylation of NFκB, in addition to evidence of mitogen-activated protein (MAP) kinase activation (pp-p38, pp-JNK, pp-42/44 extracellular signal regulating kinase (ERK)). Heat-denatured (100°C, 5 min) human recombinant eNAMPT (HD) (1 μg·mL−1, 1 h) served as a negative control confirming that eNAMPT effects do not reflect endotoxin contamination. The addition of the eNAMPT-neutralising pAb nearly totally abolished eNAMPT-induced NFκB phosphorylation and inhibited eNAMPT-induced MAP kinase activation detected by phosphorylation of ERK, p38 and JNK MAP kinases captured by densitometric measurements (n=3). c) In companion experiments, human lung ECs plated onto gold microelectrodes were challenged with either recombinant human eNAMPT alone (1 µg·mL−1), an eNAMPT-pAb mixture (eNAMPT 1 μg·mL−1 and pAb 10 μg·mL−1) or an eNAMPT-ALT-100 monoclonal antibody (mAb) mixture (eNAMPT 1 μg·mL−1 and ALT-100 10 μg·mL−1). Both eNAMPT-neutralising strategies, pAb and mAb, attenuated eNAMPT-induced declines in EC barrier integrity compared to eNAMPT alone. Human lung ECs on gold microelectrodes were also challenged with lipopolysaccharide (LPS) (1 µg·mL−1), with either PBS, eNAMPT pAb (10 µg·mL−1) or ALT-100 mAb (10 µg·mL−1) added immediately after LPS stimulation. The ALT-100 mAb, but eNAMPT pAb, also produced significant reductions in LPS-induced declines in EC barrier integrity compared to LPS alone. For trans-endothelial electrical resistance (TER) studies, normalised resistance values >1 indicate lung EC barrier enhancement, normalised resistance values <1 indicate lung EC barrier disruption. d) Bar graph quantification of the TER declines and loss of barrier integrity, where data are expressed as change in TER compared to normalised unstimulated controls at 6 h (±sem, n=3 independent experiments per condition). *: p<0.05 agonist alone versus agonist-pAb or mAb. e) Lung tissue homogenates from “one-hit” LPS-challenged (1 mg·kg−1, 18 h) with and without treatment with the eNAMPT-neutralising mAb (0.4 mg·kg−1 at time 0 h) were evaluated for Evans blue dye (EBD) accumulation in lung tissues as a reflection of extravascular dye leakage and reported as the EBD concentration (μg·g−1 lung tissue) [55]. The bar graph demonstrates that the significant LPS-induced increases in EBD accumulation are abolished by prior addition of the ALT-10 mAb. *: p<0.05 LPS versus control; #: p<0.05 LPS versus LPS+mAb. DU: densitometric units.