Fig. 5. NFATc2 is a LRRK2 kinase substrate.
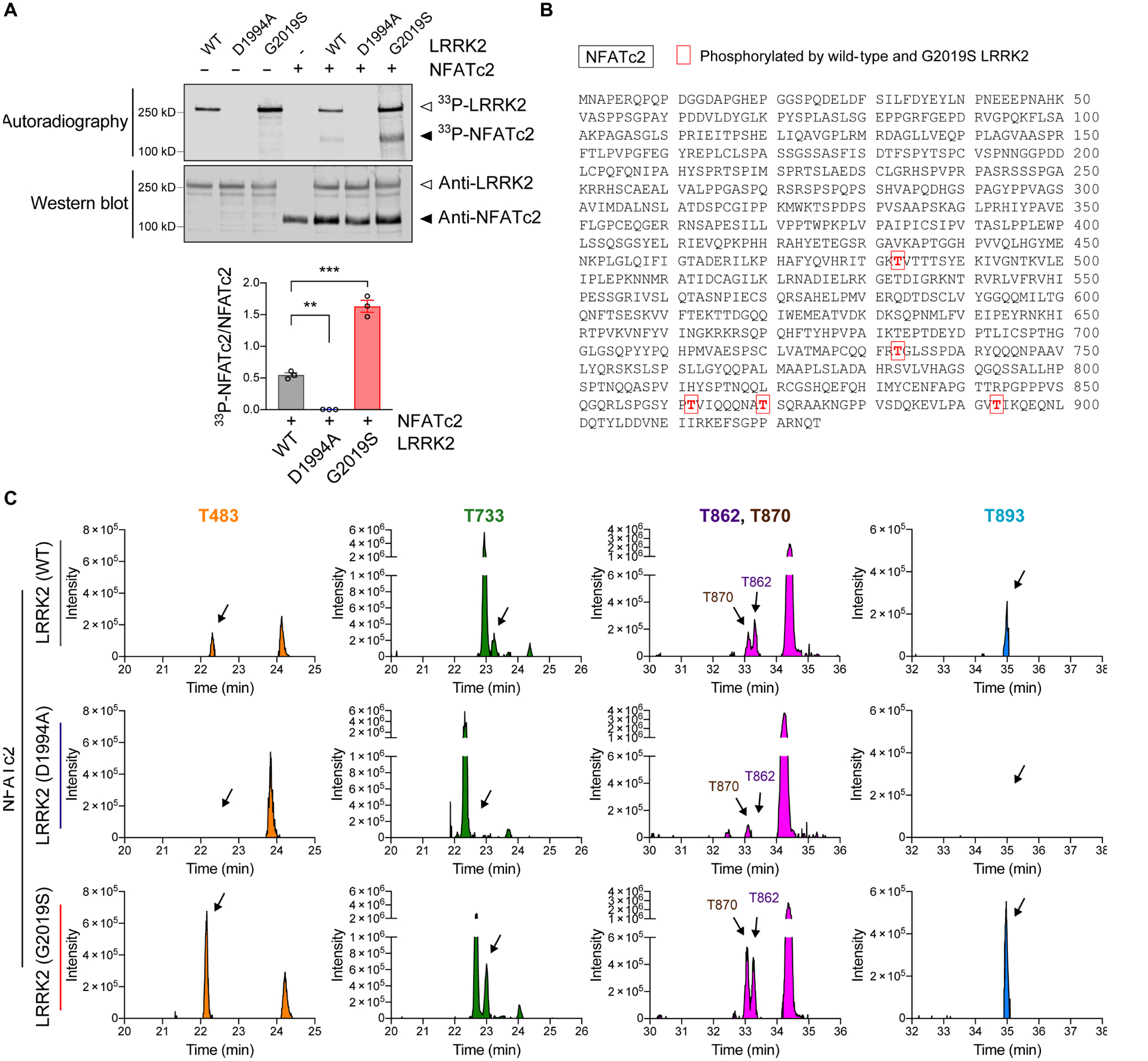
(A) In vitro LRRK2 kinase assay. Recombinant LRRK2 WT, LRRK2 D1994A, and LRRK2 G2019S were incubated in the presence or absence of NFATc2 for 30 minutes. Top: autoradiography of LRRK2 and NFATc2. Bottom: western blot analysis. The same blot was probed with anti-LRRK2 and anti-NFATc2. 33P-NFATc2 band intensity was determined by densitometric quantification and normalized to total NFATc2 on the western blot. Data are means ± SEM. *P < 0.05 and ***P < 0.001 (One-way ANOVA with Tukey’s multiple comparison post-hoc test). n = 3 per group. (B and C) Recombinant NFATc2 incubated with LRRK2 WT, LRRK2 D1994A, or LRRK2 G2019S was analyzed by LC-MS/MS. (B) Amino acid sequence of NFATc2 (NP_775114.1) and positions of 5 residues where the phosphorylation stoichiometry was increased when incubated with LRRK2 G2019S and decreased when incubated with LRRK2 D1994A relative to LRRK2 WT. (C) XIC data of phosphopeptides corresponding to the 5 phosphorylated threonine residues shown in Fig. 5b.
