Fig. 6. 3D structural analysis of NFATc2 and LRRK2 interaction.
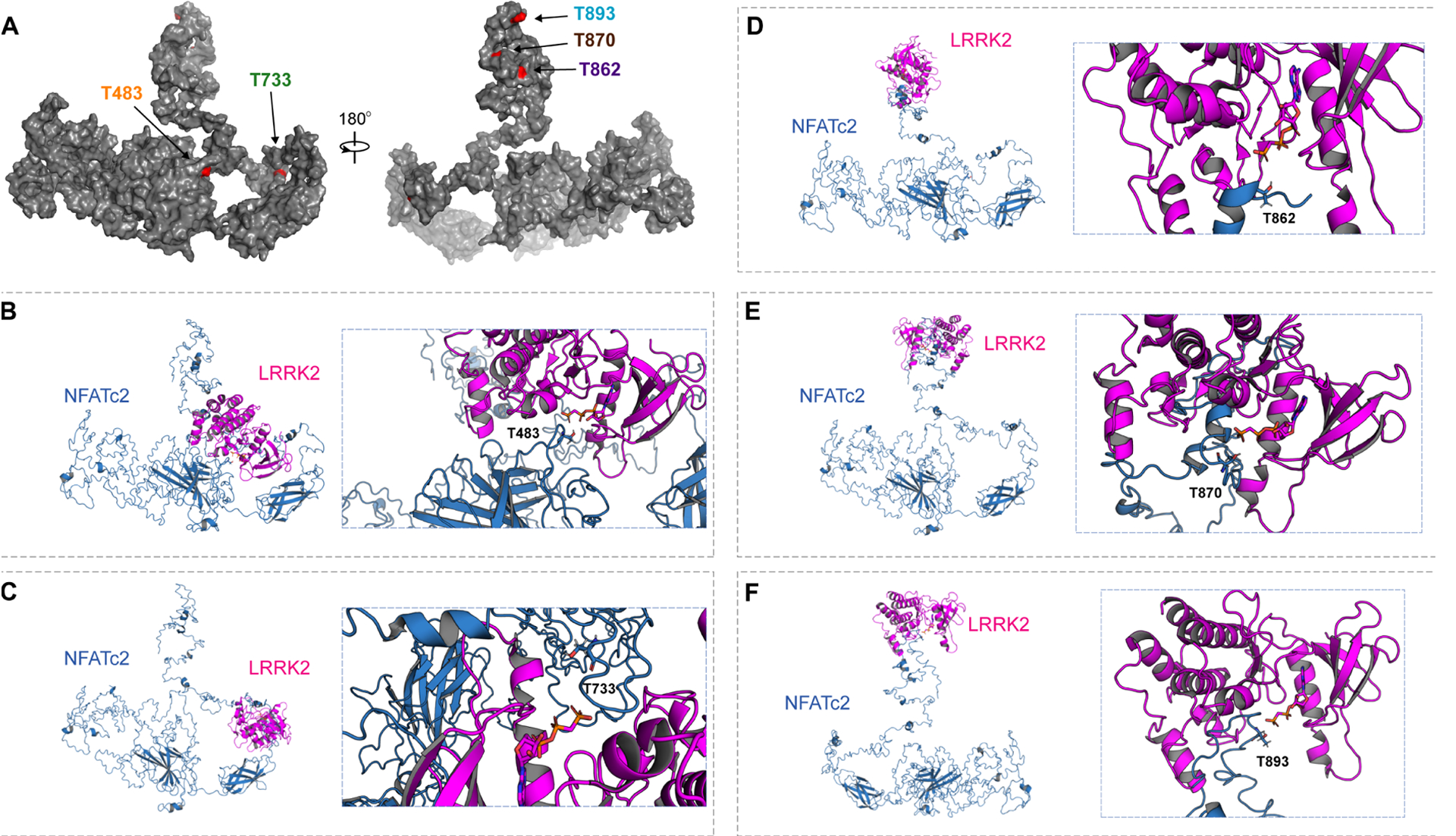
(A) A full-length structural model was generated from the crystal structure of NFATc2 from Homo sapiens (PDB: 1P7H). The five identified threonines (T) are labeled in red. (B to F) Potential kinase-substrate hetero-complex formation of LRRK2 and NFATc2. The full-length NFATc2 structure (Blue) was modeled alongside the kinase domain of the LRRK2-related protein ROCO4 from Dictyostelium discoideum (PDB: 4F0F) (Pink). The phosphate group of the kinase is labeled in orange and the target hydroxyl group on the threonine in NFATc2 is labeled in red. 3D conformations were modeled for the interactions between LRRK2 and T483 (B), T733 (C), T862 (D), T870 (E), and T893 (F).
