Abstract
Background:
When measured in adolescence or young adulthood, cardiovascular health (CVH) is associated with future subclinical CVD, but data are lacking regarding CVD events or mortality.
Objectives:
We examined associations of CVH at age 18–30 years with premature CVD and mortality.
Methods:
We analyzed data from the Coronary Artery Risk Development in Young Adults study. CVH was scored at baseline (1985–1986) using Life’s Simple 7 metrics and categorized as high (12–14 points), moderate (8–11) or low (0–7). CVD events and cause-specific mortality were adjudicated over 32 years’ follow-up. We estimated adjusted associations using Cox models and calculated event rates and population attributable fractions (PAFs) by CVH category.
Results:
Among 4,836 participants (mean age 24.9 years, 54.8% female, 50.5% Black, mean education 15.2 years), baseline CVH was high (favorable) in 28.8%, moderate in 65.0%, and low in 6.3%. During follow-up, 306 CVD events and 431 deaths occurred. The adjusted HRs (95% CI) for high (vs low) CVH were 0.14 (0.09–0.22) for CVD and 0.07 (0.03–0.19) for CVD mortality, and the PAFs for moderate/low (vs high) CVH were 0.63 (0.47–0.74) for CVD and 0.81 (0.55–0.92) for CVD mortality. Among individuals with high CVH, event rates were low across sociodemographic subgroups (e.g., CVD rates/1000 person-years: age 18–24, 0.64; age 25–30, 0.65; men, 1.04; women, 0.36; Blacks, 0.90; Whites, 0.50; ≤high-school education, 1.00; >high-school, 0.61).
Conclusions:
High CVH in late adolescence/young adulthood was associated with very low rates of premature CVD and mortality over 32 years, indicating the critical importance of maintaining high CVH.
Condensed abstract:
We investigated associations of cardiovascular health (CVH), based on Life’s Simple 7, at age 18–30 years with premature CVD and mortality over 32 years. Among 4,836 participants, baseline CVH was high (favorable) in 29%, moderate in 65%, and low in 6%. The adjusted HR for CVD was 0.14 (95% CI, 0.09–0.22) for high (vs low) CVH, and the population attributable fraction for moderate/low (vs high) CVH was 0.63 (0.47–0.74). Among individuals with high CVH, CVD rates were low across subgroups defined by age, sex, race, and education, indicating the potential impact of maintaining high CVH throughout childhood into young adulthood.
Summary Tweet:
Favorable cardiovascular health in adolescence or young adulthood was associated with very low rates of premature CVD and mortality over 32 years. Twitter handle for Senior Author: @dmljmd
Keywords: population attributable fraction, Life’s Simple 7, primordial prevention
Introduction
When measured in mid-life, cardiovascular health (CVH), as defined by the American Heart Association (AHA) using “Life’s Simple 7” metrics,(1) is associated with markedly reduced risks for cardiovascular disease (CVD), mortality, and numerous other adverse health outcomes.(2–4) CVH measured in adolescence or young adulthood has been associated with subclinical CVD,(5–10) but data are lacking regarding associations with future CVD events or mortality. Such data are needed for at least two reasons. First, available data indirectly suggest that young adult CVH may prove a key target to reduce population CVD and mortality burdens and disparities. In the majority of the US population, substantial loss of favorable (high) CVH occurs during youth,(4) and an analysis of midlife CVH found that outcomes were associated primarily with CVH at younger ages, regardless of subsequent CVH change in midlife.(11) Second, for longitudinal studies of early-life determinants of risk, CVH in late adolescence or young adulthood may be a useful intermediate or surrogate endpoint well in advance of hard clinical endpoints that would take decades to accumulate. What is needed, therefore, is quantification of late-adolescent and young-adult CVH associations with subsequent CVD events and mortality.
In this study, we analyzed >30 years of longitudinal data from the Coronary Artery Risk Development in Young Adults Study (CARDIA) to test the following two hypotheses: (1) CVH status in late adolescence or young adulthood (age 18–30 years) is associated with incident premature CVD and mortality, with very low event rates across all sociodemographic subgroups with high CVH; and (2) population attributable fractions (PAFs) for poor CVH status—i.e., the proportion that theoretically would not have occurred if all individuals had high CVH—are high for these premature events.
Methods
Study Design and Participants
CARDIA(12) is a longitudinal cohort study that began in 1985–86 with enrollment of 5,115 healthy young adults, balanced on sex, age (18–24 and 25–30 years), race (Black and White), and education (up to/through and more than high school), at four US sites: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. A total of 8 in-person follow-up examinations have occurred, most recently 30 years after baseline. Additionally, contact is maintained with participants via telephone, mail, or email every 6 months, with annual interim medical history ascertainment; over the last 5 years, >90% of the surviving cohort members have been directly contacted. The study was approved by institutional review boards at all sites, and participants gave informed consent.
CVH Measurement and Classification
The seven CVH metrics (defined as in eTable 1) were measured at baseline. Dietary intake over the past month was assessed with an interviewer-administered, quantitative diet history.(13) Because this method (versus 24-hour recalls and dietary records) is most useful for ranking individuals according to usual consumption rather than quantifying absolute intakes (i.e., estimate scales may be shifted),(14) we assessed dietary quality by ranking intakes of the 13 Healthy Eating Index-2015 (HEI-2015)(15) components to create a Relative HEI-2015 score for each participant. We retained the 13 components in the HEI-2015, except the added sugars component was replaced with sugar-sweetened beverage intake because the latter was more specifically quantified in CARDIA and is typically utilized in CVH dietary metric scoring.(1) The score range (0–5 or 0–10) for each HEI-2015 component was applied for corresponding quantiles (6 or 11 quantiles, respectively) of intakes, with higher quantiles assigned higher scores for adequacy components and lower scores for moderation components.(16) Total Relative HEI-2015 scores ranged from 0 to 100, as for the HEI-2015(15) and other AHA-recommended dietary pattern scores.(4,17) Physical activity was assessed with interviewer-administered self-report of leisure-time frequency and duration of participation in 13 specific activities over the past 12 months,(18) from which we estimated total hours per week of moderate-to-vigorous activity.(19) Cigarette smoking history was self-reported by questionnaire. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2) using standardized measurements by trained study personnel. Blood pressure (average of second and third readings) was measured after 5 minutes seated at rest. Venous blood was drawn after a 12-hour fast and analyzed for total cholesterol and glucose using standard methods.(20) Medication use for hypertension, hypercholesterolemia, and diabetes was self-reported.
We used AHA definitions(1,4) to categorize and score each of the seven CVH metrics as ideal (2 points), intermediate (1 point) or poor (0 points) and summed points across all metrics to create a total CVH score of 0 to 14 points (eTable 1). We classified total CVH scores as high (12–14 points), moderate (8–11) or low (0–7), consistent with prior studies.(21,22) In sensitivity analyses, we (1) classified individuals with a CVH score of 12 but with diabetes or smoking as having moderate CVH, rather than high CVH, given the considerable risk conferred by these factors, and (2) applied alternative thresholds for blood pressure classification according to the 2017 American College of Cardiology (ACC)/AHA Guidelines(23) (Supplemental Table 1 footnote).
CVD and Mortality Outcomes
The primary outcome of interest was incident CVD, including fatal or nonfatal myocardial infarction (MI), coronary revascularization (non-elective), heart failure, stroke, transient ischemic attack, hospitalized unstable angina, carotid or peripheral arterial disease requiring intervention, or other fatal heart or atherosclerotic disease. Secondary outcomes included the composite of CVD or all-cause mortality, as well as individual outcomes of CVD mortality (including fatal coronary or other heart disease, stroke, or other definite atherosclerotic disease as adjudicated, underlying causes of death), all-cause mortality, MI, coronary revascularization, heart failure, and stroke. Events were reported by participants during annual telephone interviews (with specific inquiry regarding hospitalizations). Deaths were identified on an ongoing basis from family contacts and National Death Index queries; vital status follow-up is thus virtually complete on all participants. Reported events were validated and adjudicated by two members of the CARDIA endpoints committee through medical record review using standard definitions.(24) For the current analysis, adjudication of events was complete through 2017–18, 32 years after baseline. Given the participants’ baseline mean age (24.9 years) and age range (18–30 years), all events were considered premature.
Covariates
Age, sex, race, and highest attained educational level (total years achieved across follow-up, as some participants were still pursuing education at baseline at age 18–30 years) were self-reported on questionnaires and included as covariates for adjustment. In a sensitivity analysis, we also adjusted for income 5 years after baseline (not available at baseline in CARDIA).
Statistical Analysis
For the current analyses, we excluded 1 participant who withdrew consent, 2 participants who were transgender, 132 participants with extreme reported caloric intakes (>8000 or <800 kcal/day for men; >6000 or <600 kcal/day for women), and 144 participants who were missing data for one or more of the CVH metrics, for a total analytic sample of 4,836 participants.
Descriptive statistics were calculated. We also calculated crude incidence rates for each outcome across CVH categories. After confirming appropriateness of proportional hazards assumptions, we used Cox proportional hazards regression to estimate hazards ratios (HR) for associations between baseline CVH score or category and incident CVD and mortality outcomes, adjusting for age, sex, race, and education (in years). We also generated Cox adjusted cumulative incidence curves.
We estimated the PAFs of moderate, low, and combined moderate/low (vs high) CVH categories for each outcome over 30 years’ follow-up. The PAF estimates the proportion of outcome events attributable to a risk factor—in this case, moderate or low CVH—out of all events in the population over a certain time interval. To calculate PAF, we used a recently developed method and program that accounts for death as a competing risk.(25–27) The calculation takes into account both the baseline prevalence of the CVH category (moderate or low) in the study population and the strengths of the associations between moderate or low (vs high) CVH and the outcomes (CVD event and death). The strengths of the associations were estimated using piecewise constant hazards models adjusted for baseline age, sex, race, and highest education. PAFs and 95% confidence intervals (CI) were estimated using piecewise constant hazard models with complementary logarithmic transformation.(26,27)
All analyses were repeated in subgroups defined a priori by baseline age (18–24 and 25–30 years), sex, race, and total education level (up to/through high school [≤HS] and beyond high school [>HS]); to preserve sample size, further stratification (e.g., by race-sex group) was not performed. A multiplicative interaction term testing for significant differences in associations of CVH with outcomes across the four race-sex groups (Black men and women, White men and women) was tested in the fully adjusted Cox models and yielded no evidence of effect modification for any outcome. In a secondary analysis, we examined associations between the levels of each of the seven CVH metrics and the primary outcome of CVD using Cox proportional hazards regression with adjustment for age, sex, race, education, and the levels (ideal, intermediate, or poor) of the other CVH metrics. For all analyses, we used SAS version 9.4 (SAS Institute, Cary, NC), with a two-sided significance level of 0.05.
Results
Analytic Sample
Baseline characteristics and CVH levels of the 4,836 included participants are shown in Tables 1 and 2. The mean age was 24.9 (SD 3.6) years and the mean years of education were 15.2 (2.6), with 54.8% women and 50.5% Black participants. CVH was generally more favorable among participants with more than high school education (versus up to/through high school education), among Whites (versus Blacks), and to a lesser extent among those aged 18–24 years (versus 25–30 years) and among women (versus men).
Table 1.
Baseline Participant Characteristics
| Overall | Age | Sex | Race | Education | |||||
|---|---|---|---|---|---|---|---|---|---|
| 18–24 Years | 25–30 Years | Men | Women | Black | White | ≤ HS | > HS | ||
| N (%) | 4836 | 2135 (44.1) | 2701 (55.9) | 2186 (45.2) | 2650 (54.8) | 2441 (50.5) | 2395 (49.5) | 972 (20.1) | 3864 (79.9) |
| Age, years | 24.9 (3.6) | 21.4 (2.0) | 27.7 (1.7) | 24.9 (3.6) | 24.9 (3.7) | 24.4 (3.8) | 25.4 (3.4) | 24.3 (3.8) | 25.0 (3.6) |
| Female, N (%) | 2650 (54.8) | 1165 (54.6) | 1485 (55.0) | - | - | 1383 (56.7) | 1267 (52.9) | 454 (46.7) | 2196 (56.8) |
| Black, N (%) | 2441 (50.5) | 1242 (58.2) | 1199 (44.4) | 1058 (48.4) | 1383 (52.2) | - | - | 665 (68.4) | 1776 (46.0) |
| >HS Education, N (%) | 3864 (79.9) | 1651 (77.3) | 2213 (81.9) | 1668 (76.3) | 2196 (82.9) | 1776 (72.8) | 2088 (87.2) | - | - |
| Total Education, years* | 15.2 (2.6) | 14.9 (2.5) | 15.5 (2.7) | 15.1 (2.7) | 15.3 (2.6) | 14.4 (2.3) | 16.1 (2.7) | 11.7 (0.7) | 16.1 (2.2) |
| HEI-2015 Diet Score | 50 (14) | 47 (13) | 53 (14) | 47 (12) | 53 (14) | 47 (12) | 54 (14) | 44 (12) | 52 (13) |
| Physical Activity, hrs/wk† | 2.3 (2.3) | 2.5 (2.3) | 2.2 (2.2) | 2.9 (2.5) | 1.8 (1.9) | 2.2 (2.3) | 2.4 (2.3) | 2.0 (2.1) | 2.4 (2.3) |
| Cigarettes per day | 0 (0–10.0) | 0 (0–7.0) | 0 (0–10.0) | 0 (0–10.0) | 0 (0–10.0) | 0 (0–7.0) | 0 (0–10.0) | 5.0 (0–15.0) | 0 (0–7.0) |
| Body Mass Index, kg/m2 | 24.5 (5.0) | 24.1 (4.9) | 24.8 (5.1) | 24.4 (3.9) | 24.6 (5.8) | 25.3 (5.7) | 23.6 (4.1) | 24.7 (5.3) | 24.4 (5.0) |
| Systolic BP, mm Hg | 110 (11) | 110 (10) | 110 (11) | 115 (10) | 107 (10) | 111 (11) | 109 (11) | 112 (11) | 110 (11) |
| Diastolic BP, mm Hg | 69 (10) | 67 (9) | 70 (10) | 71 (10) | 67 (9) | 69 (10) | 68 (9) | 68 (11) | 69 (9) |
| BP Medication Use | 108 (2.2) | 19 (0.9) | 89 (3.3) | 43 (2.0) | 65 (2.5) | 76 (3.1) | 32 (1.3) | 32 (3.3) | 76 (2.0) |
| Total Cholesterol, mg/dL | 177 (33) | 172 (33) | 181 (34) | 176 (35) | 177 (33) | 178 (34) | 176 (32) | 176 (36) | 177 (33) |
| HDL Cholesterol, mg/dL | 53 (13) | 52 (13) | 54 (14) | 50 (13) | 56 (13) | 54 (13) | 52 (13) | 52 (14) | 53 (13) |
| LDL Cholesterol, mg/dL | 109 (31) | 106 (30) | 112 (31) | 110 (32) | 108 (31) | 110 (32) | 109 (30) | 108 (33) | 110 (31) |
| Triglycerides, mg/dL | 73 (48) | 69 (39) | 76 (54) | 80 (57) | 67 (37) | 66 (37) | 79 (56) | 77 (53) | 72 (47) |
| Lipid Medication Use | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fasting Glucose, mg/dL | 83 (16) | 82 (14) | 83 (17) | 84 (14) | 81 (17) | 82 (19) | 83 (12) | 83 (16) | 82 (16) |
| Diabetes Medication Use | 40 (0.8) | 12 (0.6) | 28 (1.0) | 12 (0.6) | 28 (1.1) | 27 (1.1) | 13 (0.5) | 11 (1.1) | 29 (0.8) |
Continuous variables are shown as mean (SD) or median (IQR); categorical variables are shown as N (%).
Education is the total attained across follow-up, as some participants were still pursuing education at the time of the baseline examination at age 18–30 years.
Hours per week of moderate-to-vigorous leisure-time activity.
BP, blood pressure; HDL, high-density lipoprotein; HEI, Healthy Eating Index; HS, high school; hrs, hours; LDL, low-density lipoprotein; wk, week.
Table 2.
Baseline Cardiovascular Health Status
| Overall | Age | Sex | Race | Education | |||||
|---|---|---|---|---|---|---|---|---|---|
| 18–24 Years | 25–30 Years | Men | Women | Black | White | ≤HS | >HS | ||
| Diet | |||||||||
| Ideal | 97 (2.0) | 27 (1.3) | 70 (2.6) | 10 (0.5) | 87 (3.3) | 21 (0.9) | 76 (3.2) | 6 (0.6) | 91 (2.4) |
| Intermediate | 3644 (75.4) | 1478 (69.2) | 2166 (80.2) | 1548 (70.8) | 2096 (79.1) | 1704 (69.8) | 1940 (81.0) | 590 (60.7) | 3054 (79.0) |
| Poor | 1095 (22.6) | 630 (29.5) | 465 (17.2) | 628 (28.7) | 467 (17.6) | 716 (29.3) | 379 (15.8) | 376 (38.7) | 719 (18.6) |
| Physical Activity | |||||||||
| Ideal | 1820 (37.6) | 879 (41.2) | 941 (34.8) | 1046 (47.9) | 774 (29.2) | 877 (35.9) | 943 (39.4) | 316 (32.5) | 1504 (38.9) |
| Intermediate | 2361 (48.8) | 1021 (47.8) | 1340 (49.6) | 954 (43.6) | 1407 (53.1) | 1165 (47.7) | 1196 (49.9) | 468 (48.2) | 1893 (49.0) |
| Poor | 655 (13.5) | 235 (11.0) | 420 (15.6) | 186 (8.5) | 469 (17.7) | 399 (16.4) | 256 (10.7) | 188 (19.3) | 467 (12.1) |
| Smoking | |||||||||
| Ideal | 3125 (64.6) | 1402 (65.7) | 1723 (63.8) | 1394 (63.8) | 1731 (65.3) | 1530 (62.7) | 1595 (66.6) | 423 (43.5) | 2702 (69.9) |
| Intermediate | 280 (5.8) | 118 (5.5) | 162 (6.0) | 121 (5.5) | 159 (6.0) | 114 (4.7) | 166 (6.9) | 53 (5.5) | 227 (5.9) |
| Poor | 1431 (29.6) | 615 (28.8) | 816 (30.2) | 671 (30.7) | 760 (28.7) | 797 (32.7) | 634 (26.5) | 496 (51.0) | 935 (24.2) |
| Body Mass Index | |||||||||
| Ideal | 3157 (65.3) | 1473 (69.0) | 1684 (62.4) | 1406 (64.3) | 1751 (66.1) | 1431 (58.6) | 1726 (72.1) | 611 (62.9) | 2546 (65.9) |
| Intermediate | 1111 (23.0) | 443 (20.8) | 668 (24.7) | 603 (27.6) | 508 (19.2) | 602 (24.7) | 509 (21.3) | 229 (23.6) | 882 (22.8) |
| Poor | 568 (11.8) | 219 (10.3) | 349 (12.9) | 177 (8.1) | 391 (14.8) | 408 (16.7) | 160 (6.7) | 132 (13.6) | 436 (11.3) |
| Blood Pressure* | |||||||||
| Ideal | 3648 (75.4) | 1659 (77.7) | 1989 (73.6) | 1365 (62.4) | 2283 (86.2) | 1775 (72.7) | 1873 (78.2) | 701 (72.1) | 2947 (76.3) |
| Intermediate | 1083 (22.4) | 451 (21.1) | 632 (23.4) | 748 (34.2) | 335 (12.6) | 600 (24.6) | 483 (20.2) | 242 (24.9) | 841 (21.8) |
| Poor | 105 (2.2) | 25 (1.2) | 80 (3.0) | 73 (3.3) | 32 (1.2) | 66 (2.7) | 39 (1.6) | 29 (3.0) | 76 (2.0) |
| Total Cholesterol | |||||||||
| Ideal | 3727 (77.1) | 1750 (82.0) | 1977 (73.2) | 1693 (77.5) | 2034 (76.8) | 1839 (75.3) | 1888 (78.8) | 744 (76.5) | 2983 (77.2) |
| Intermediate | 898 (18.6) | 323 (15.1) | 575 (21.3) | 388 (17.8) | 510 (19.3) | 488 (20.0) | 410 (17.1) | 176 (18.1) | 722 (18.7) |
| Poor | 211 (4.4) | 62 (2.9) | 149 (5.5) | 105 (4.8) | 106 (4.0) | 114 (4.7) | 97 (4.1) | 52 (5.4) | 159 (4.1) |
| Glucose | |||||||||
| Ideal | 4684 (96.9) | 2086 (97.7) | 2598 (96.2) | 2114 (96.7) | 2570 (97.0) | 2362 (96.8) | 2322 (97.0) | 930 (95.7) | 3754 (97.2) |
| Intermediate | 123 (2.5) | 39 (1.8) | 84 (3.1) | 61 (2.8) | 62 (2.3) | 62 (2.5) | 61 (2.6) | 34 (3.5) | 89 (2.3) |
| Poor | 29 (0.6) | 10 (0.5) | 19 (0.7) | 11 (0.5) | 18 (0.7) | 17 (0.7) | 12 (0.5) | 8 (0.8) | 21 (0.5) |
| Total CVH Score† | 10.3 (1.8) | 10.5 (1.7) | 10.2 (1.8) | 10.3 (1.7) | 10.4 (1.8) | 10.0 (1.8) | 10.7 (1.7) | 9.5 (1.8) | 10.5 (1.7) |
| CVH Category | |||||||||
| High | 1391 (28.8) | 646 (30.3) | 745 (27.6) | 590 (27.0) | 801 (30.2) | 505 (20.7) | 886 (37.0) | 132 (13.6) | 1259 (32.6) |
| Moderate | 3141 (65.0) | 1394 (65.3) | 1747 (64.7) | 1463 (66.9) | 1678 (63.3) | 1739 (71.2) | 1402 (58.5) | 717 (73.8) | 2424 (62.7) |
| Low | 304 (6.3) | 95 (4.5) | 209 (7.7) | 133 (6.1) | 171 (6.5) | 197 (8.1) | 107 (4.5) | 123 (12.7) | 181 (4.7) |
Data are shown as N (%).
Using the primary blood pressure definition, as shown in eTable 1.
Range of CVH scores, 0–14 points.
CVH, cardiovascular health; HS, high school.
Crude Incidence Rates
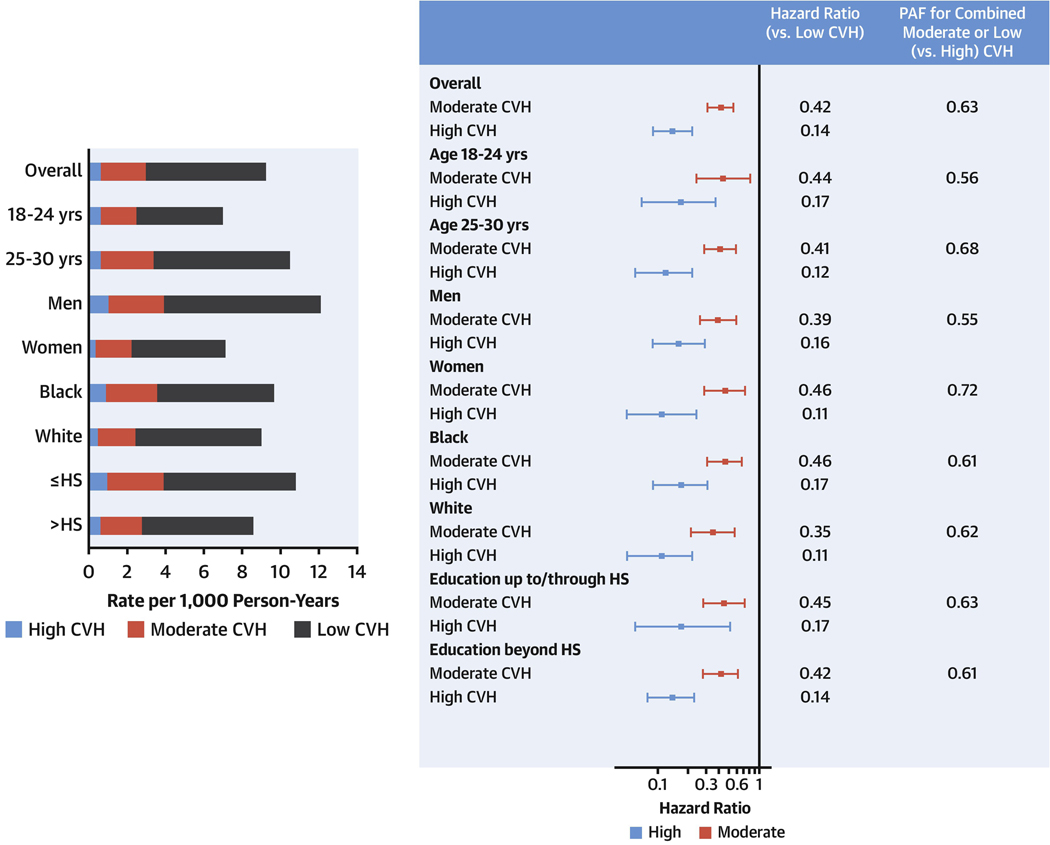
During a median follow-up duration of 31.9 years, 306 incident CVD events (at a mean age of 48.7 [SD 6.9] years; 100%/100% at age <65, 97%/98% at age <60, and 82%/81% at age <55 years among men/women) and 431 all-cause deaths (at a mean age of 45.9 [SD 9.8] years) occurred overall (eTable 2 shows outcomes by CVH category). Incidence rates for CVD and mortality outcomes were very low among individuals with high CVH as compared with rates among individuals with moderate or low CVH, both overall and across sociodemographic subgroups (Table 3, Figure 1, eTables 3–6). For example, among individuals with low CVH, CVD rates were 6.27 per 1000 person-years overall and ranged from 4.51 (age 18–24 years) to 8.16 (men) per 1000 person-years among sociodemographic subgroups. Among individuals with high CVH, incident CVD rates were 0.64 per 1000 person-years overall and ranged from 0.36 (women) to 1.04 (men) per 1000 person-years.
Table 3.
Event Rates, Adjusted* Hazard Ratios, and Population Attributable Fractions for Incident Premature Cardiovascular Disease† Events and Mortality, Overall
| N of Events | Crude Incidence Rate per 1,000 Person-Years | Adjusted* Hazard Ratio (95% CI), versus Low CVH | Population Attributable Fraction (95% CI), versus High CVH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low CVH | Moderate CVH | High CVH | Moderate CVH | High CVH | Moderate CVH | Low CVH | Moderate/Low CVH | ||
| CVD† | 306 | 6.27 | 2.34 | 0.64 | 0.42 (0.31–0.57) | 0.14 (0.09–0.22) | 0.48 (0.34–0.59) | 0.15 (0.11–0.19) | 0.63 (0.47–0.74) |
| CVD† Mortality | 123 | 3.08 | 0.93 | 0.11 | 0.40 (0.26–0.61) | 0.07 (0.03–0.19) | 0.60 (0.41–0.73) | 0.21 (0.13–0.28) | 0.81 (0.55–0.92) |
| All-Cause Mortality | 431 | 7.15 | 3.19 | 1.30 | 0.55 (0.42–0.73) | 0.30 (0.21–0.44) | 0.32 (0.18–0.43) | 0.10 (0.07–0.13) | 0.42 (0.26–0.54) |
| CVD† or All-Cause Mortality | 628 | 10.71 | 4.76 | 1.84 | 0.52 (0.41–0.65) | 0.25 (0.18–0.34) | 0.36 (0.26–0.45) | 0.11 (0.08–0.13) | 0.47 (0.35–0.56) |
| Myocardial Infarction | 155 | 3.71 | 1.14 | 0.28 | 0.32 (0.21–0.48) | 0.08 (0.04–0.17) | 0.51 (0.33–0.64) | 0.19 (0.13–0.25) | 0.70 (0.49–0.83) |
| Coronary Revascularization | 90 | 2.23 | 0.63 | 0.21 | 0.27 (0.16–0.46) | 0.09 (0.04–0.20) | 0.45 (0.20–0.62) | 0.20 (0.11–0.28) | 0.65 (0.34–0.81) |
| Heart Failure | 78 | 1.55 | 0.59 | 0.16 | 0.47 (0.26–0.85) | 0.19 (0.07–0.48) | 0.43 (0.07–0.65) | 0.14 (0.04–0.22) | 0.57 (0.12–0.79) |
| Stroke | 105 | 1.88 | 0.81 | 0.23 | 0.51 (0.30–0.87) | 0.19 (0.08–0.42) | 0.46 (0.18–0.65) | 0.12 (0.05–0.19) | 0.59 (0.25–0.77) |
Adjusted for field center, sex, age, race, and education (total cumulative, in years).
CVD includes myocardial infarction, heart failure, stroke, transient ischemic attack, hospitalized unstable angina, carotid or peripheral arterial disease requiring intervention, or other fatal heart or atherosclerotic disease. For CVD death, underlying causes of death included coronary or other heart disease, stroke, or other definite atherosclerotic disease.
CI, confidence interval; CVD, cardiovascular disease; CVH, cardiovascular health.
Figure 1. Unadjusted Incident Premature CVD and Mortality Rates by Baseline CVH Category.
Incidence rates were very low during long-term follow-up among individuals with high baseline cardiovascular health (CVH) compared with rates among individuals with low or moderate baseline CVH, both overall and across sociodemographic subgroups. Cardiovascular disease (CVD) includes myocardial infarction, heart failure, stroke, transient ischemic attack, hospitalized unstable angina, carotid or peripheral arterial disease requiring intervention, or other fatal heart or atherosclerotic disease. For CVD death, underlying causes of death included coronary or other heart disease, stroke, or other definite atherosclerotic disease. HS, high school education.
Associations between Baseline CVH and Incident Premature CVD and Mortality
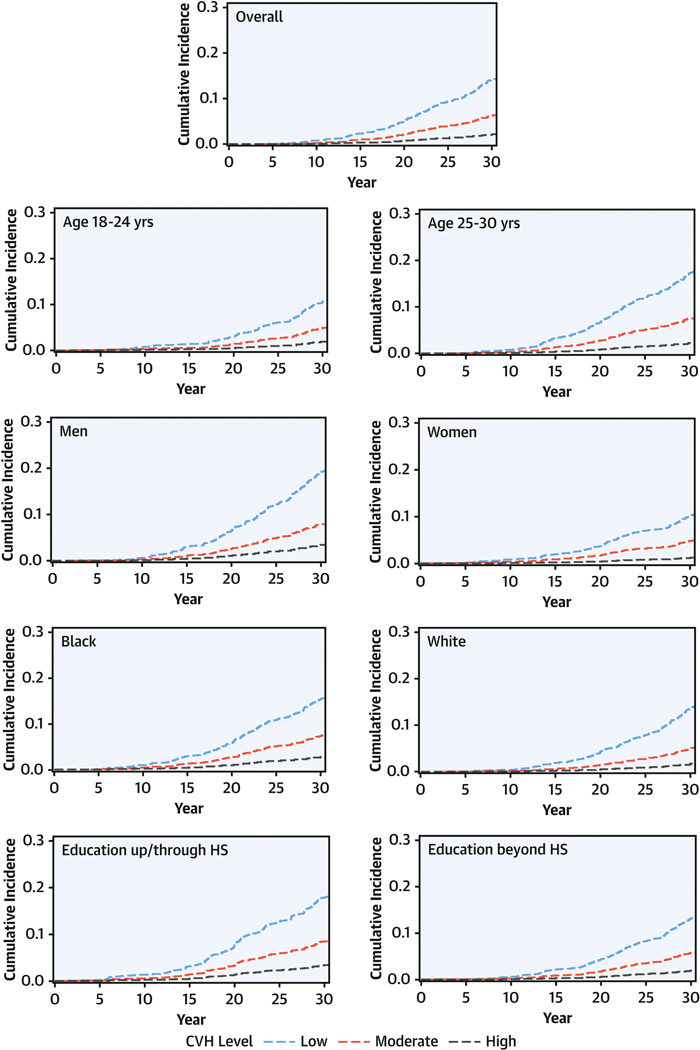
In the overall sample, more favorable CVH was significantly associated with lower risks for CVD, mortality, and each CVD subtype (Table 3, Figures 2–3). For the continuous CVH score, the adjusted HR (95% CI) per each 1-point higher CVH score was 0.73 (0.68–0.77) for incident CVD events, and ranged from 0.69 for CVD mortality to 0.80 for all-cause mortality. There were similar findings for all event subtypes (Figure 3). When analyzing categorical levels of CVH, moderate (vs low) CVH was significantly associated with 45% to 73% lower adjusted hazards across all outcomes, and high (vs low) CVH was associated with even lower risk, with 70% to 93% lower adjusted hazards (Table 3). Across sociodemographic subgroups, patterns of association for CVH status were similar, although event numbers were low for some outcomes (Figures 2–3, Supplemental Tables 3-6).
Figure 2. Adjusted CVD Cumulative Incidence Curves by CVH Level at Age 18–30 Years.
In Cox models adjusted for field center, sex, age, race, and total education, more favorable cardiovascular health (CVH) was associated with significantly lower probability of cardiovascular disease (CVD) over 30 years, overall and across sociodemographic subgroups. CVD was defined as in Figure 1. HS, high school.
Figure 3. Adjusted Associations between Baseline CVH Score (per 1 Point Higher) and Incident Premature CVD and Mortality.

In Cox models adjusted for field center, sex, age, race, and total education, each 1-point higher baseline cardiovascular health (CVH) score was associated with 20–31% lower hazards for incident premature cardiovascular disease (CVD) and mortality. Findings were similar across sociodemographic subgroups. CVD and CVD death were defined as in Figure 1. Edu, education; HR, hazard ratio; HS; high school; yrs, years.
In sensitivity analyses, when participants with diabetes or current smoking were not eligible for “high CVH” status (Supplemental Table 7), when a more restrictive blood pressure classification based on the 2017 ACC/AHA guideline(23) was utilized (Supplemental Table 8), or when additional adjustment for income was performed (Supplemental Table 9), associations of CVH with outcomes were similar to those in the primary analyses.
Population Attributable Fractions for CVD and Mortality by CVH Category
The PAF (95% CI) for CVD associated with combined moderate/low CVH compared with high CVH was 0.63 (0.47–0.74), suggesting that 63% of these premature CVD events would not have happened if all participants had high CVH at baseline. PAFs were significant and high across all events, ranging from 0.42 for premature all-cause mortality to 0.81 for premature CVD mortality (Table 3). PAFs for moderate CVH (alone, vs high CVH) ranged from 0.32 to 0.60 across event types. PAFs for low CVH (alone, vs high CVH) were smaller, ranging from 0.10 to 0.21 across event types, given the low prevalence of low CVH in this young, generally healthy cohort. Across sociodemographic subgroups (Supplemental Tables 3-6), PAFs were not significantly different.
Secondary Analysis: Adjusted Associations of Individual CVH Metrics with CVD
Five of the seven metrics (all but diet and physical activity) were independently associated with CVD after adjustment for sociodemographics and the levels of all other CVH metrics (Supplemental Table 10).
Discussion
Principal Findings
In this community-based, biracial population of 4,836 late adolescents and young adults who were followed for >30 years, several significant findings emerged. First, while this was a younger, asymptomatic, and generally healthy cohort by most standards, more than two-thirds of participants had moderate or worse CVH and only approximately one-fourth had high CVH levels at age 18–30 years. Second, among those with high CVH at baseline, rates of premature CVD events and all-cause mortality were very low (less than 0.2% per year) during long-term follow-up. Third, CVH status in late adolescence/young adulthood was significantly associated with risks for incident premature CVD events and mortality, with lower hazards by 20–31% per each 1-point higher baseline CVH score. Those with categorical high compared with low CVH at baseline had 70–93% lower risks for premature CVD events or all-cause mortality. Fourth, PAFs for moderate/low (vs high) baseline CVH were high, at 63% for CVD and up to 81% for CVD mortality, suggesting that the vast majority of premature events could have been avoided if all participants had high CVH at baseline. Finally, we observed no significant differences in patterns of findings across sociodemographic subgroups, including sex and race subgroups.
Findings in the Context of Prior Literature
Several prior studies have quantified the association of mid-life CVH with later-life CVD events and mortality, and these suggested significant age effects whereby associations were strongest when CVH was measured in younger compared with older middle age. The most recent meta-analysis examining incident CVD events included 12 prospective studies with 210,443 adults at a mean age of 59 years.(28) The pooled HRs (95% CI) for CVD among those with high CVH and intermediate CVH vs low CVH (each defined somewhat differently than in the present study) were 0.23 (0.13–0.34) and 0.45 (0.31–0.58), respectively, with some variability by CVD event subtype. An inverse relationship was observed between the mean age at which CVH was measured (between 52 and 73 years across studies) and the strength of the association with outcomes.(28) In a meta-analysis examining mortality only, including 6 prospective studies with 146,454 adults at mean ages of 46–69 years, the pooled HRs (95% CI) for high CVH vs low CVH (again defined somewhat differently than the present study) were 0.54 (0.41–0.69) for all-cause mortality and 0.30 (0.18–0.51) for CVD mortality.(29) For CVD mortality, associations were significantly stronger when CVH was measured at age <50 years versus ≥50 years.
Fewer studies have examined CVH status at young ages, but these have demonstrated that CVH measured in adolescence or young adulthood is associated with later subclinical CVD, including high-risk carotid and aortic intima-media thickness(5–8) and left ventricular hypertrophy and diastolic dysfunction.(10) Although intima-media thickness,(30,31) left ventricular hypertrophy,(32) and diastolic dysfunction(33,34) are each associated with CVD events and mortality, quantification of the direct relationship of CVH status at young ages with hard clinical outcomes has been lacking. The current analysis of 4,638 adults at a mean age of 25 years provides unique evidence that the relative associations of late-adolescent/young-adult CVH with CVD and mortality appear to be at least as strong as, and possibly stronger than, those for mid-life and later CVH reviewed above. Furthermore, the CVD and mortality events in the current study were premature, occurring in mid-life (<65 years). This deserves emphasis because recent data suggest that rates of such premature heart disease death (i.e., in adults aged 45–64 years) have increased in the US during 2011 to 2017,(35) and the implications of CVH for an individual’s healthy life-expectancy in particular (or “healthspan”) may be greatest when CVH is measured at younger (vs older) ages.
We also found that CVH measured at age 18–30 years was associated with PAFs for CVD (0.63) and especially for CVD mortality (0.81) that are at least as high as, and in some cases higher than, those for CVH measured in mid-life. In a prior report of data from 13,541 adults at a mean baseline age of 60 years, baseline smoking, obesity, hypertension, hypercholesterolemia, and diabetes mellitus (5 of the 7 CVH metrics) had a combined PAF of 0.53 (95% CI, 0.47–0.58) for incident CVD over 10 years of follow-up.(36) For mortality, based upon data from 16,215 US adults at a mean age of 45 years, the PAFs (95% CI) for non-ideal CVH (i.e., <7/7 metrics ideal) were 0.64 (0.28–0.84) for CVD mortality and 0.59 (0.33–0.76) for all-cause mortality.(3) Significant effect modification with age was detected such that PAFs for CVD mortality were higher when CVH was measured at age <60 vs ≥60 years (0.90 [0.07–0.99] vs 0.44 [0.10–0.83], interaction P=.016). This age dependency aligns with the finding of higher PAFs for CVD and mortality outcomes associated with baseline CVH in our study population with baseline ages of 18–30 years.
Implications
Thus, the first major implication of the current study is that preservation of high CVH into late adolescence/young adulthood merits further study as a key target for reducing population burdens of, and disparities in, CVD and mortality. The high PAFs associated with CVH at age 18–30 years in the current study correlated with both a high prevalence of moderate/low CVH at baseline and strong associations between CVH and outcomes, suggesting that both primordial prevention of CVH declines during youth and improved detection and treatment of established or emerging risk factors in late adolescence and young adulthood are needed. In the US, CVH levels drop precipitously with age such that the prevalence of high CVH (≥5/7 metrics ideal) is already just 45% at age 12–19 years, declining further to 32% at age 20–39 years, and 10.6% at age 40–59 years.(4) National racial and socioeconomic disparities in these CVH declines were reflected in our study by somewhat less favorable CVH levels in Blacks (vs Whites) and in individuals with education up to/through (vs beyond) high school at age 18–30 years. Other data suggest that social determinants of health must be addressed as root causes of risk factor development and poor control, as well as independent contributors to adverse outcomes.(37,38)
Nevertheless, in the current study, event rates were exceedingly low over >30 years of follow-up across all sociodemographic subgroups when high CVH was present at age 18–30 years. Preservation of CVH through youth into early adulthood may thus be particularly favorable for subsequent prognosis, meriting special focus on primordial prevention throughout childhood and adolescence by public health and pediatric health care initiatives. For young adults with established risk factors, improved systems of healthcare delivery are needed. The 18- to 30-year-old age group is particularly difficult to engage clinically due to gaps in insurance coverage and discontinuity from pediatric to adult care (alongside other major life transitions). Undertreatment of young-adult risk factors is further compounded by clinical guidelines that focus primarily on adults ≥40 years of age or utilize 10-year CVD risk calculators heavily weighted on age.(39) Together, these factors result in low rates of ambulatory care use, preventive health guideline adherence, and awareness, treatment, and control of established risk factors among young adults in the current system.(39–43)
Another major implication of our data is that CVH in late adolescence or young adulthood may serve as a valid intermediate or surrogate outcome for investigations of early-life determinants of premature CVD and mortality risks. Given retention, funding, and various other issues for pediatric studies that take decades to accumulate hard outcomes, such a strong late-adolescent/young-adult predictor of future events is likely to be valuable in pediatric research.
Strengths and Limitations
Key strengths of this study include the >30-year follow-up of a unique age group and the community-based design with a high proportion of Black participants. This study also had limitations. First, given the young baseline age of participants, only premature events have been captured thus far. Second, a single measurement of CVH (rather than change over time) was utilized in the models; however, the strong associations observed for CVH measured once in young adulthood (despite the likely subsequent CVH losses) highlight the relevance of this period in the life course. Third, no correction was performed for multiple testing, and event numbers were low for some outcomes, particularly for CVD subtypes in subgroup analyses; thus, although results were largely consistent, subgroup analyses should be interpreted cautiously. Fourth, diet and physical activity levels were self-reported; despite use of rigorous, interviewer-administered methods, the resulting imprecision(14,44) along with limited event numbers likely contributed to lack of independent statistical significance for these metrics in the association with CVD. Fifth, despite multivariable adjustment, residual confounding (e.g., due to unmeasured socioeconomic variables) is possible.
Conclusions
In a community-based, biracial cohort of late adolescents and young adults, high CVH at age 18–30 years was strongly associated with low rates of incident premature CVD and mortality over >30 years of follow-up, overall and in all sociodemographic subgroups. Furthermore, moderate and low CVH status in late adolescence and young adulthood had high PAFs for premature CVD events and mortality, indicating the importance of defining strategies to maintain high CVH throughout childhood into young adulthood.
Supplementary Material
Central Illustration. Implications of Cardiovascular Health at Age 18 to 30 Years for Incident Premature Cardiovascular Disease Over >30 Years.
In the bar graph (top), unadjusted cardiovascular disease (CVD) incidence rates were very low among individuals with high baseline cardiovascular health (CVH) in all sociodemographic subgroups. In the forest plot (bottom left), after adjustment for field center, sex, age, race, and total education, categorically moderate (vs low) CVH was associated with 58% lower hazard for CVD, and high (vs low) CVH was associated with 86% lower hazard for CVD. The population attributable fraction (bottom right) of CVD was 63% for moderate/low (vs high) baseline CVH. Findings were similar across sociodemographic subgroups. CVD was defined as in Figure 1. Edu, education; HR, hazard ratio; HS, high school; yrs, years.
Clinical Perspectives.
Competencies in Medical Knowledge:
Life’s Simple 7 factors – a healthy diet, physical activity, non-smoking, body mass index, blood pressure, total cholesterol, and fasting glucose — together contribute to cardiovascular (CV) health. Optimal levels of these factors between the ages of 18 and 30 years is associated with a markedly reduced risk of developing CV disease and mortality later in life. Despite differences in the prevalence of CV health related to age, sex, race and education, most premature CV disease and mortality could be prevented if the risk profile of the entire population were optimized.
Translational Outlook:
Further research is needed to define strategies that optimize risk factors and preserve CV health through childhood and young adulthood.
Acknowledgements:
The authors gratefully acknowledge the contributions of the many people whose collaborative efforts run the CARDIA study. We also thank the participants of the CARDIA study for their long-term commitment to the study and important contributions to science. This manuscript has been reviewed by CARDIA for scientific content.
Funding Sources: CARDIA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), Kaiser Foundation Research Institute (HHSN268201800004I), and the Johns Hopkins University School of Medicine (HHSN268200900041C). AMP’s work was supported in part by career development award K23 HL145101 from the NHLBI. SSK’s work was supported in part by an award from the NIH/NHLBI and National Center for Advancing Translational Sciences (KL2TR001424) and an award from the American Heart Association (19TPA34890060).
Abbreviations and acronyms:
- ACC
American College of Cardiology
- AHA
American Heart Association
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- CVD
cardiovascular disease
- CVH
cardiovascular health
- HEI
Health Eating Index
- HR
hazard ratio
- MI
myocardial infarction
- PAF
population attributable fraction
Footnotes
Disclosures: The authors report no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2.Folsom AR, Yatsuya H, Nettleton JA et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Cogswell ME, Dana Flanders W et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among us adults. JAMA 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Benjamin EJ et al. Heart Disease and Stroke Statistics-2020 Update. Circulation 2020;141:e139-e596. [DOI] [PubMed] [Google Scholar]
- 5.Laitinen TT, Pahkala K, Magnussen CG et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood. Circulation 2012;125:1971–8. [DOI] [PubMed] [Google Scholar]
- 6.Pahkala K, Hietalampi H, Laitinen TT et al. Ideal cardiovascular health in adolescence. Circulation 2013;127:2088–96. [DOI] [PubMed] [Google Scholar]
- 7.Oikonen M, Laitinen TT, Magnussen CG et al. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT. J Am Heart Assoc 2013;2:e000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen NB, Krefman AE, Labarthe D et al. Cardiovascular Health Trajectories From Childhood Through Middle Age and Their Association With Subclinical Atherosclerosis. JAMA Cardiol 2020;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen TT, Ruohonen S, Juonala M et al. Ideal cardiovascular health in childhood-Longitudinal associations with cardiac structure and function. Int J Cardiol 2017;230:304–309. [DOI] [PubMed] [Google Scholar]
- 10.Desai CS, Ning H, Liu K et al. Cardiovascular Health in Young Adulthood and Association with Left Ventricular Structure and Function Later in Life. J Am Soc Echocardiogr 2015;28:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Sloten TT, Tafflet M, Perier MC et al. Association of Change in Cardiovascular Risk Factors With Incident Cardiovascular Events. JAMA 2018;320:1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP et al. CARDIA. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 13.McDonald A, Van Horn L, Slattery M et al. The CARDIA dietary history. J Am Diet Assoc 1991;91:1104–12. [PubMed] [Google Scholar]
- 14.Thompson FE, Subar AF. Chapter 1-Dietary Assessment Methodology. In: Coulston AM, Boushey CJ, Ferruzzi MG, Delahanty LM, editors. Nutrition in the Prevention and Treatment of Disease (Fourth Edition): Academic Press, 2017:5–48. [Google Scholar]
- 15.Krebs-Smith SM, Pannucci TE, Subar AF et al. Update of the Healthy Eating Index. J Acad Nutr Diet 2018;118:1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K, Daviglus ML, Loria CM et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age. Circulation 2012;125:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US Adults, 1999–2012. JAMA 2016;315:2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidney S, Jacobs DR Jr., Haskell WL et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol 1991;133:1231–45. [DOI] [PubMed] [Google Scholar]
- 19.Gooding HC, Ning H, Gillman MW et al. Application of a Lifestyle-Based Tool to Estimate Premature Cardiovascular Disease Events in Young Adults. JAMA Intern Med 2017;177:1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutter GR, Burke GL, Dyer AR et al. Cardiovascular risk factors in young adults. Control Clin Trials 1991;12:1S-77S. [DOI] [PubMed] [Google Scholar]
- 21.Polonsky TS, Ning H, Daviglus ML et al. Association of Cardiovascular Health With Subclinical Disease and Incident Events. J Am Heart Assoc 2017;6:e004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd-Jones DM. Cardiovascular Health Among Pregnant Women, Aged 20 to 44 Years, in the United States. J Am Heart Assoc 2020;9:e015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Hypertension 2018;71:e13-e115. [Google Scholar]
- 24.CARDIA Endpoint Events Manual of Operations. http://www.cardia.dopm.uab.edu/images/more/recent/CARDIA_Endpoint_Events_MOO_v03_20_2015.pdf. Published 2015. Accessed May 19, 2020.
- 25.Benichou J A review of adjusted estimators of attributable risk. Stat Methods Med Res 2001;10:195–216. [DOI] [PubMed] [Google Scholar]
- 26.Laaksonen MA, Harkanen T, Knekt P, Virtala E, Oja H. Estimation of population attributable fraction for disease occurrence in a cohort study design. Stat Med 2010;29:860–74. [DOI] [PubMed] [Google Scholar]
- 27.Laaksonen MA, Virtala E, Knekt P, Oja H, Harkanen T. SAS Macros for Calculation of Population Attributable Fraction in a Cohort Study Design. Journal of Statistical Software 2011;43. [Google Scholar]
- 28.Ramirez-Velez R, Saavedra JM, Lobelo F, Celis-Morales CA, del Pozo-Cruz B, Garcia-Hermoso A. Ideal Cardiovascular Health and Incident Cardiovascular Disease Among Adults. Mayo Clin Proc 2018;93:1589–1599. [DOI] [PubMed] [Google Scholar]
- 29.Guo LL, Zhang SS. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality. Clin Cardiol 2017;40:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Oord SC, Sijbrands EJ, ten Kate GL et al. Carotid intima-media thickness for cardiovascular risk assessment. Atherosclerosis 2013;228:1–11. [DOI] [PubMed] [Google Scholar]
- 31.Hodis HN, Mack WJ, LaBree L et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998;128:262–9. [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. New Engl J Med 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 33.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the Risk of Progression From Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure. JACC Heart Fail 2016;4:237–48. [DOI] [PubMed] [Google Scholar]
- 34.Desai CS, Colangelo LA, Liu K et al. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults. Am J Epidemiol 2013;177:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtin SC. Trends in cancer and heart disease death rates among adults aged 45–64. National Vital Health Statistics Reports 2019;68:1–9. [PubMed] [Google Scholar]
- 36.Cheng S, Claggett B, Correia AW et al. Temporal trends in the population attributable risk for cardiovascular disease. Circulation 2014;130:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havranek EP, Mujahid MS, Barr DA et al. Social determinants of risk and outcomes for cardiovascular disease. Circulation 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 38.Whitaker KM, Jacobs DR Jr., Kershaw KN et al. Racial Disparities in Cardiovascular Health Behaviors. Am J Prev Med 2018;55:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gooding H, Johnson HM. The Unchartered Frontier. Curr Cardiovasc Risk Rep 2016;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fortuna RJ, Robbins BW, Halterman JS. Ambulatory care among young adults in the United States. Ann Intern Med 2009;151:379–85. [DOI] [PubMed] [Google Scholar]
- 41.Bucholz EM, Gooding HC, de Ferranti SD. Awareness of Cardiovascular Risk Factors in U.S. Young Adults Aged 18–39 Years. Am J Prev Med 2018;54:e67-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Moran AE. Trends in the Prevalence, Awareness, Treatment, and Control of Hypertension Among Young Adults in the United States, 1999 to 2014. Hypertension 2017;70:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyre AD, Muntner P, Menke A, Raggi P, He J. Trends in ATP-III-defined high blood cholesterol prevalence, awareness, treatment and control among U.S. adults. Ann Epidemiol 2007;17:548–55. [DOI] [PubMed] [Google Scholar]
- 44.Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr 2015;6:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.