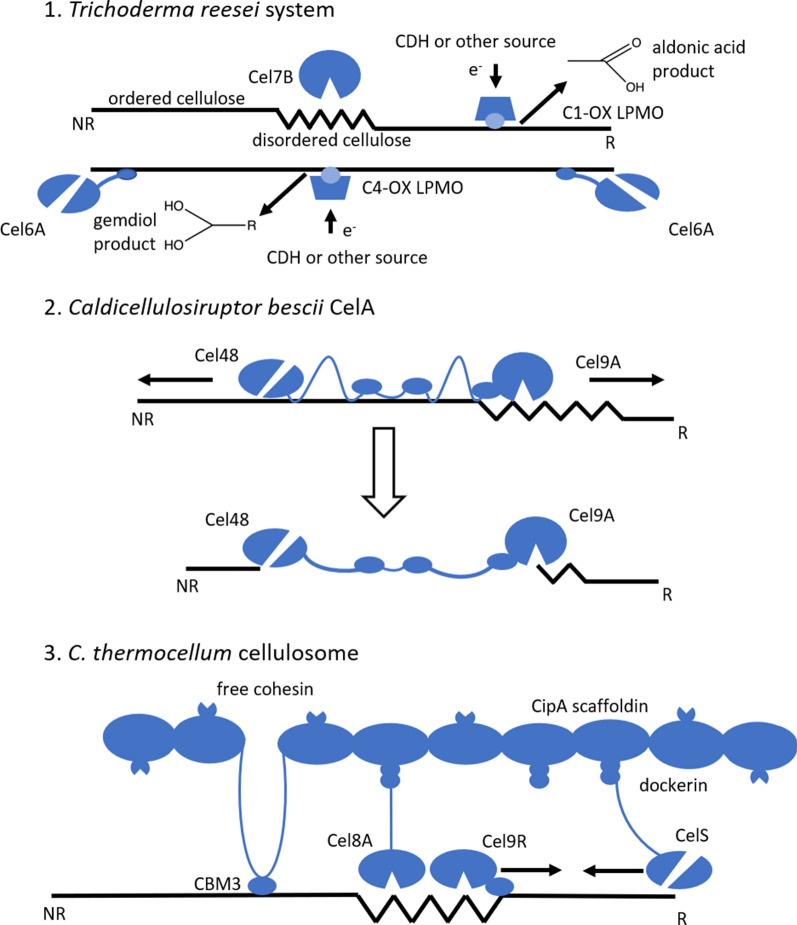
Fig. 8.
Shown are model representations of the (1) Mono-functional system found in cellulolytic fungi (i.e., Trichoderma reesei): Cel6A and Cel7A are processive cellobiohydrolase that initiate from the non-reducing and reducing ends of cellulose chains, respectively. Cel7B is an endoglucanase which hydrolyzes cellulose at mid-chain positions thus producing new chain ends for cellobiohydrolases to initiate hydrolysis. CDH is a cellobiose dehydrogenase which acts as a redox partner in the LPMO mechanism. (2) The multi-functional system used by some cellulolytic bacteria (i.e., Caldicellulosiruptor bescii) consists of a GH9 endoglucanase catalytic domain and a GH48 exoglucanase domain and also contains three cellulose-binding domains. This bacterial cellulase is one of the most effective enzyme systems ever reported for degrading cellulose and exhibits a “pit-digging” mechanism as shown in reference [203]. (3) The highly aggregated cellulosome consisting of various cellulase and cellulose-binding domains bound to a protein scaffold by the dockerin–cohesin interaction. This diagram represents the canonical Clostridium thermocellum CipA scaffolding structure containing nine type I cohesin domains (type II cohesin domains are not shown). Depicted are a CBM3 cellulose-binding domain, a Cel8A endoglucanase, and a CelS exoglucanase. This system works synergistically with free enzymes such as Cel9I, a processive endoglucanase. A detailed description of these enzyme systems is presented in reference [178–180]