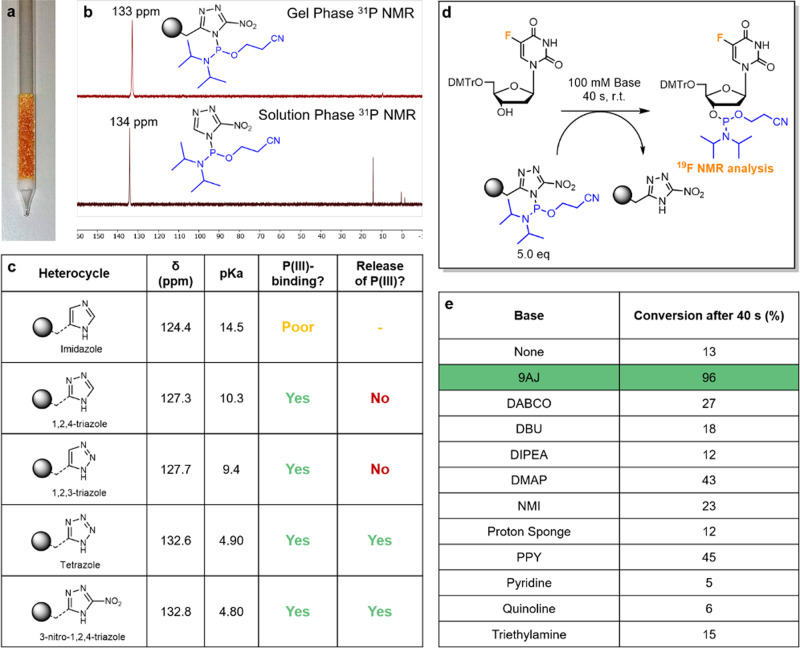
Fig. 2. Optimization of the solid-phase azole and base additives.
Initial study of resin-bound P((III) species with different azoles. a Picture of the modified NMR tube (figure by Alexander F. Sandahl). b Stacked spectra of resin-bound and solution phase P(III)-nitrotriazolide. c Summary of resin-bound azoles ability to bind and release P(III) upon elution with MeOH as measured by 31P NMR analysis. d Scheme depicting transfer of P(III) to alcohol using five equivalents resin-bound P(III) in the modified NMR tube with floxuridine as screening substrate for 19F NMR analysis. e Summary of conversion obtained after 40 s reaction time with various bases based on the amounts of product and starting material by 19F NMR analysis. 9AJ (highlighted in green) gave the highest yield for this study. 9AJ 9-azajulolidine, DABCO 1,4-diazabicyclo[2.2.2]octane, DBU 1,8-diazabicyclo[5.4.0]undec-7-ene, DIPEA N,N-diisopropylethylamine, DMAP 4-dimethylaminopyridine, NMI N-methylimidazole, PPY 4-pyrrolidinopyridine.