Abstract
Resin-based composite has overtaken dental amalgam as the most popular material for the repair of lost or damaged tooth structure. In spite of the popularity, the average composite lifetime is about half that of amalgam restorations. The leading cause of composite-restoration failure is decay at the margin where the adhesive is applied. The adhesive is intended to seal the composite/tooth interface, but the adhesive seal to dentin is fragile and readily degraded by acids, enzymes and other oral fluids. The inherent weakness of this material system is attributable to several factors including the lack of antimicrobial properties, remineralization capabilities and durable mechanical performance ― elements that are central to the integrity of the adhesive/dentin (a/d) interfacial seal. Our approach to this problem offers a transition from a hybrid to a biohybrid structure. Discrete peptides are tethered to polymers to provide multi-bio-functional adhesive formulations that simultaneously achieve antimicrobial and remineralization properties. The bio-additive materials design combines several functional properties with the goal of providing an adhesive that will serve as a durable barrier to recurrent decay at the composite/tooth interface. This article provides an overview of our multi-faceted approach which uses peptides tethered to polymers and new polymer chemistries to achieve the next generation adhesive system ― an adhesive that provides antimicrobial properties, repair of defective dentin and enhanced mechanical performance.
Keywords: Bio-additives, Adhesive Design, Peptide Engineering, Antimicrobial Peptide, Mineralization, Polymer Chemistry
1. Introduction
According to a global burden disease study, dental caries which affected 3.5 billion people globally with untreated caries in 2017 is one of the most prevalent health problem 1–7. Resin-based composites are among the most commonly used materials to restore form and function to teeth damaged by decay8–9 however, the clinical lifetime of composite-restorations can be as low as 5 to 7 years10. The problem of repeated composite restoration replacement is pervasive—nearly 70% of all composite restorations are replacements for failed resin restorations10. Repeated dental-restoration replacement risks pulpal injury, increased tooth weakness, and eventually, total tooth loss11.
The leading cause of composite-restoration failure is recurrent marginal decay. In brief, restoration of the tooth surface involves removal of decay, acid-etching of enamel and dentin, application of dental adhesive, and finally, restoring of form and function using composite restorative material. The composite is too viscous to bond directly to the tooth―a lower viscosity adhesive is used to bond the composite to the tooth structure. The infiltration of the adhesive into the acid-etched dentin, i.e. the demineralized dentin collagen, is termed hybridization, and the resulting structure has been named the “hybrid layer”12–14. The ideal hybrid layer is described as the demineralized dentin collagen completely encased in adhesive, but this ideal structure has not been achieved in vivo4, 8, 15–16. This failure is attributed, in part, to a discrepancy between the depth of adhesive infiltration and the depth of demineralized dentin collagen3, 8, 15, 17–20. In addition to the hybrid layer, other factors that affect the overall quality of the a/d interfacial seal include the clinical substrate (variation in composition, heterogeneous structure, caries-affected dentin, sclerotic dentin, etc.), adhesive composition, operator technique, moisture contamination, and patient characteristics21.
The oral cavity is a caustic environment that challenges the durability and integrity of the most dental resins. To improve the compatibility between the dental adhesive and the wet, demineralized dentin matrix, hydrophilic and ionic monomers have been incorporated in contemporary dental adhesives 22. The increase in hydrophilic components facilitates adhesive infiltration, however there are several disadvantages including increased water sorption which weakens the polymer. Water plasticizes the polymer and promotes chemical hydrolysis of the adhesive 19. Salivary esterases23–30 and esterases from Streptococcus mutans30 may accelerate this hydrolysis process leading to long-term release of degradation by-products. The by-products accumulate at the a/d interface and increase the virulence of cariogenic bacteria, e.g. S. mutans, provoking a degradative positive-feedback loop.
Adhesion of the cariogenic bacteria, S. mutans, to the tooth/adhesive/composite interface creates a microenvironment that promotes the subsequent attachment and growth of bacteria and biofilms. Lactic acid produced by S. mutans demineralizes the tooth surface, acid as well as enzymes produced by S. mutans erode the dental adhesive, and together these activities lead to wider and deeper gaps at the margin between the tooth and composite. The gaps provide an ideal environment for bacteria to proliferate which leads ultimately to recurrent decay and failure of the composite restoration20, 31–33.
1. Bio-additive hybrid dental adhesives
Dental adhesives possess broad and versatile properties, but they lack the bioactivity that is associated with native structures including biomolecules. Incorporating peptides with specific biological functionalities, e.g. antimicrobial and remineralization properties, as part of the material system could enhance the durability and integrity of the adhesive and the seal formed at the a/d interface. Polymer-peptide conjugates are generally hybrid soft materials, which are designed to achieve synergistic behavior of both components while overcoming the disadvantages inherent to the individual components34–35. Over the past two decades, studies of the polymer-peptide conjugates have ranged from fundamental science to biomedical and nonbiological applications35–43. To date, the majority of these polymer-peptide conjugates have been soft, rapidly eroding hydrogel-based materials that degrade or clear after a few weeks in vivo.44 The relatively low mechanical properties and rapid erosion of these conjugates inhibit their application as dental restorative materials. Designing the conjugates to achieve their full potential is still a major challenge for biomedical applications.
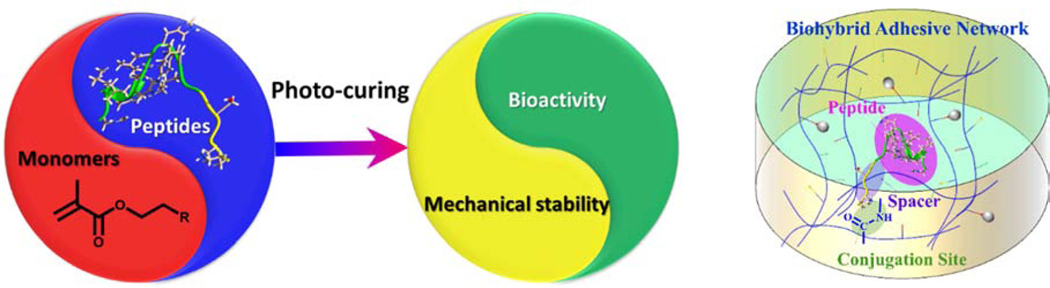
Our recent investigations have led to a synergistic approach to the design and development of a bio-additively designed hybrid dental adhesive. As Fig. 1 illustrates, this biohybrid design is promising for achieving superior performance while regaining the integrity of the tooth structure within the a/d interface. The antimicrobial and remineralization activities are enabled by the peptides conjugated to the polymer while new polymer chemistries lead to enhanced mechanical properties via an autonomous strengthening reaction. The polymer-peptide conjugation provides relatively high antimicrobial activity and promising remineralization of dentin at the a/d surface. The polymer alone does not inherently possess antimicrobial properties or remineralization capabilities. The novel polymer chemistries enhance the mechanical behavior through a mechanism that provides intrinsic reinforcement of the polymer network in both neutral and acidic conditions45.
Fig. 1.
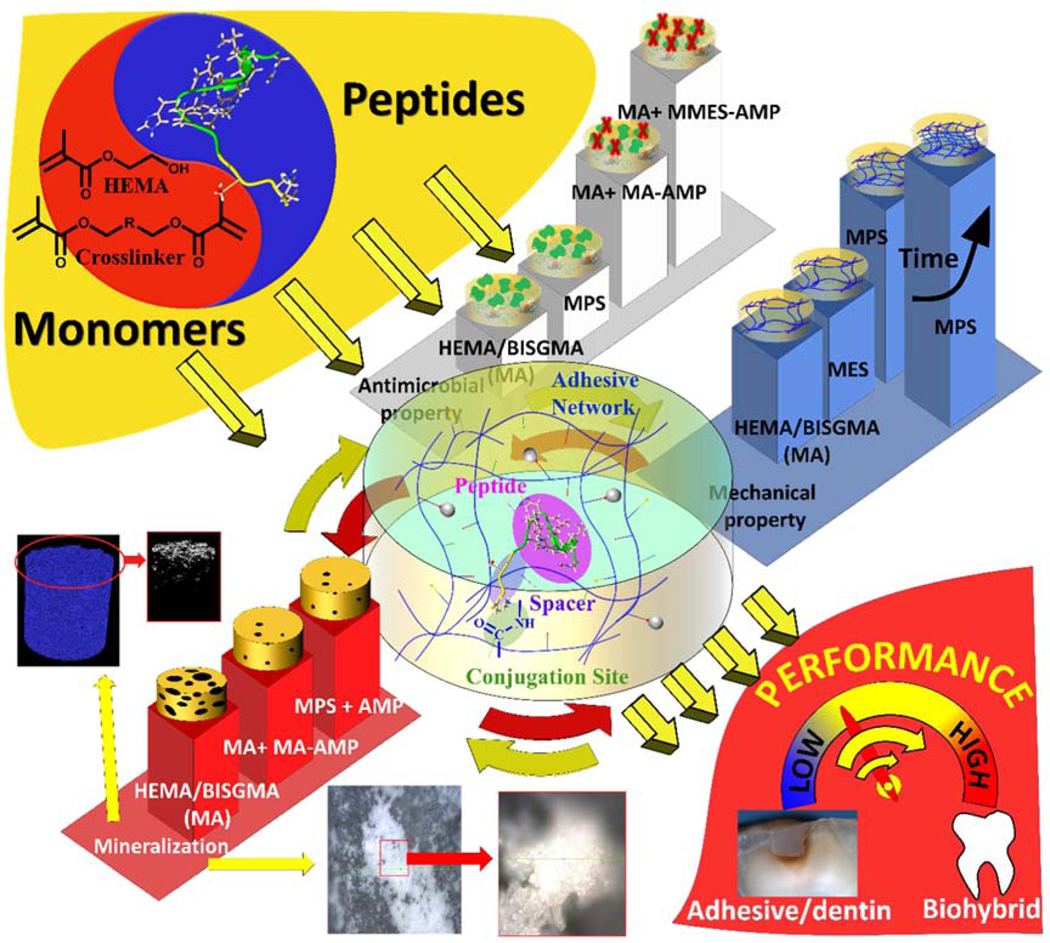
Bio-additive hybrid material design strategy leading to high performance a/d interface.
2. Adhesive/dentin interface as the weak link
In spite of improvements in dental adhesive technology, the integrity of the a/d interface is vulnerable to degradation under the caustic conditions present in the mouth. The integrity of the a/d interface and the durability of the bonds formed at this interface have been linked directly to the quality of the hybrid layer. The characteristics of the etched dentin surface, structural and compositional heterogeneity of the hybrid layer and the physicochemical properties of adhesives have been summarized to clarify their effects on the integrity of the a/d interface 46–47.
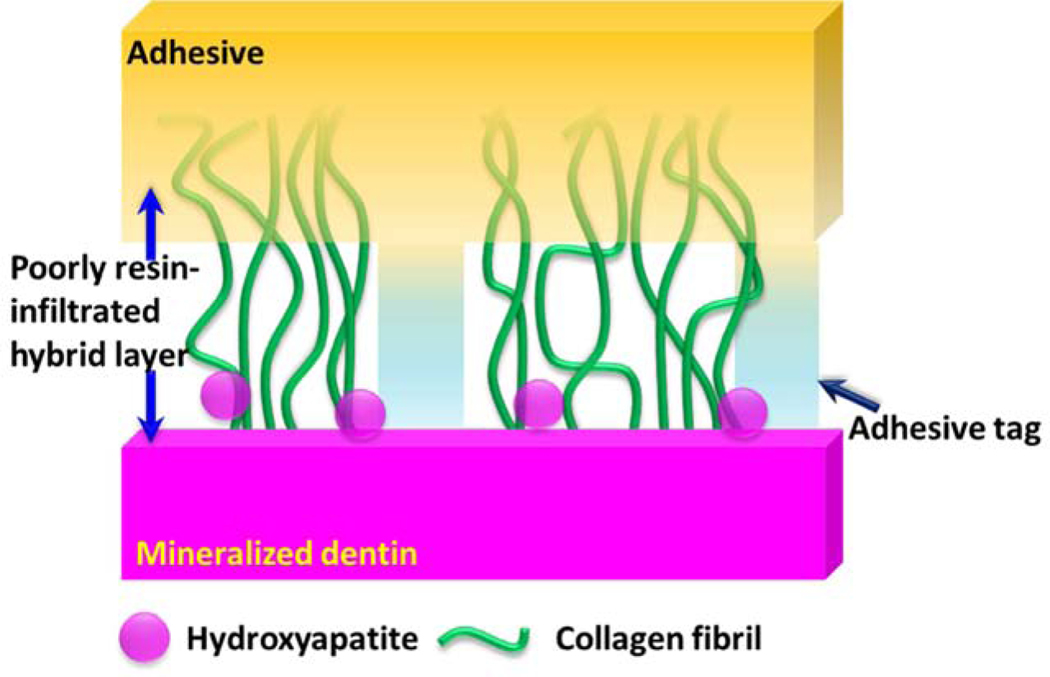
The demineralized dentin collagen matrix acts as the scaffold for the resin infiltration. Due to the varied amphiphilicity of the components in the dental adhesives and the fluid filled demineralized dentin matrix, adhesive infiltration into the collagen matrix results in an imperfect hybrid layer15, 48–49 as shown in Fig. 2. The imperfect hybrid layer leads to exposed collagen fibrils. The inferior properties of the exposed collagen as compared to resin-infiltrated or mineralized collagen50 lead to collagen that is not protected against challenges that can provoke denaturation and early failure51. The limited durability of the imperfect hybrid layer shortens the lifetime of tooth-colored resin-based restorations.
Fig. 2.
Non-ideal a/d interface exhibiting heterogeneity in terms of composition of the hybrid layer and adhesive throughout the depth of the demineralized dentin collagen.
3. Tuning the antimicrobial property of dental adhesive with peptide conjugation
Antimicrobial peptides (AMPs) have been widely recognized as existing in all life forms as part of the immune systems to fight infections. Large databases, such as the Antimicrobial Peptide Database52 and LAMP database,53 identify thousands of naturally occurring AMPs. All known taxa produce AMPs54–58 which often serve as part of the innate immune response. In addition to naturally occurring peptides, synthetically-designed peptides are needed to address drug resistance in pathogens without leading to reduction of the efficacy of naturally produced AMPs. Recently, AMPs have been engineered to have superior bactericidal characteristics and broad-spectrum activity59–61.
AMPs have been studied in various dental applications such as coating agents for implants62–63 and additives for adhesive materials64–65 to combat pathogenic microorganisms66. Despite these advances, successful commercial applications that realize the vast potential of AMPs are quite limited in dentistry. Using high concentrations of AMPs through systemic delivery raises toxicity concerns, showing the need for an alternative delivery strategy. Another issue is non-specific interactions between AMPs and polymers―these non-specific interactions may limit the peptides’ availability, causing reduced antibacterial efficacy. 65, 67–68
Strategies to conjugate peptides to polymers, which are referred as the hybrid constructs, involve different coupling chemistries combining defined monomer and amino acids sequences. Our approach involves tethering peptides with distinctive bioactivities site specifically to monomers using an oligomeric spacer group to form peptide-monomer pairs. Next, these peptide-monomer pairs were copolymerized and the resulting polymers exhibited antimicrobial and remineralization properties simultaneously. Different strategies that have been pursued by other groups include an exploration of the interdependence of linked components. The interdependence has been studied by tuning the component’s physical properties to optimize the biologic functionalities 43, 69–70.
In tissue engineering71–72 and in surgical wound dressings,73–75 AMPs have been successfully incorporated into hydrogels via conjugation. Robust antimicrobial activity has been demonstrated with AMP-hydrogels developed from either natural or synthetic materials39, 71, 76–77. Despite successful antimicrobial efficacy, currently developed natural or synthetic hydrogels with antibiotic functionality showed limited application in dentistry due to poor mechanical strength. The typical compression moduli of studied AMP-hydrogel conjugates varies from about 0.1 to ~40 kPa71, 76, 78–83, which are not suitable for use in dental restorations. The reported Young’s modulus of hydrated commercial dental adhesives ranges from 0.5 to 4 GPa at 37°C84–85. Until recently, no polymer material has been developed to combine the mechanical strength necessary to serve as a dental adhesive and provide antimicrobial activity from AMPs80.
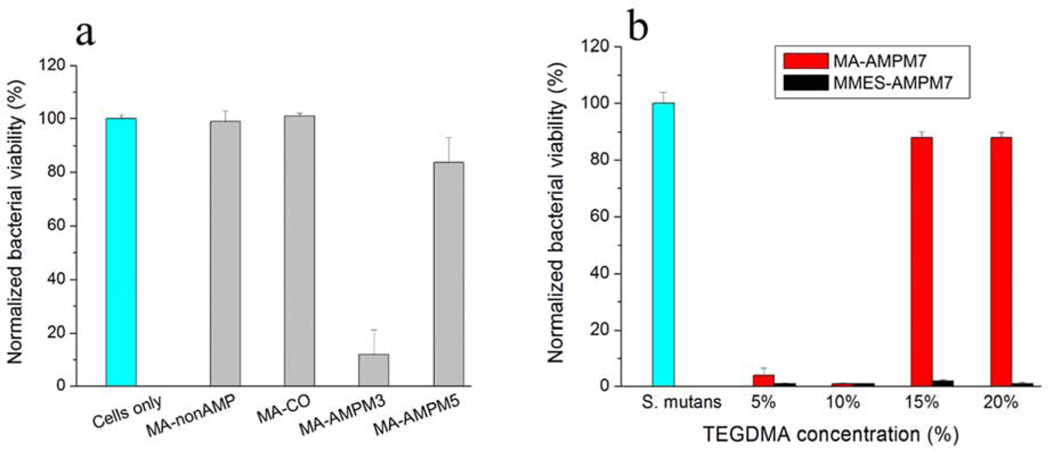
Peptide diffusion in the resin could be a challenging as peptide may have restricted conformation limiting its activity due to non-specific adsorptions55, 67. To prevent this, we incorporated an AMP sequence specifically conjugated to a commonly used monomer for dental adhesive formulation (Fig. 3). The antimicrobial peptides GH12 and AMP2 were selected with their well-known activities against and reduction of cariogenic virulence factors of S. mutans 62, 86–88,89. We designed engineered derivatives from GH12 and AMP2 with an addition of a spacer sequence. The α-NH2 of lysine (K) in both GH12 and AMP2 peptide derivatives was used to react with –COOH of methacrylic acid (MA) or mono-2-(methacryloyloxy) ethyl succinate (MMES) for the synthesis of peptide-monomers (Table 1). Both MA and MMES have one carboxylic acid group for peptide conjugation and one C=C bond for copolymerization with the polymer matrix. The difference between MMES and MA is the chain length and the flexibility. ε-NH2 of lysine (K) was blocked before cleavage and ε-NH2 of lysine (K) can be used for conjugation of other functional groups. To provide the conformational flexibility between the peptide and the polymer matrix, a spacer domain is introduced. Several tailored AMP-monomers have been synthesized using GH12 and AMP2 derivative sequences to enable subsequent methacrylate conjugation. The spacer-integrated antimicrobial peptides were linked to MA or MMES and the resulting MA-AMP or MMES-AMP monomers were then copolymerized into dental adhesives. Among the two different spacer sequences investigated for the AMP-adhesive polymer sets, the ones with GGG spacer demonstrated significantly more activity compared to the ones with SSSGGG spacer (Table 1 and Fig. 4(a))62, 90–91,92–93.
Fig. 3.
Tethering peptides to polymers leading to superior antimicrobial property and mechanical performance.
Table 1.
The sequence information of the AMPs and AMP-monomers with their antimicrobial properties
| AMP and AMP-monomer | Sequence | MIC (μg/mL) |
|---|---|---|
| GH12 (COOH) | GLLWHLLHHLLH (COOH) | 15.6 |
| AMPM1 | K_GGGSG_GLLWHLLHHLLH (COOH) | 31.3 |
| AMPM3 | K_GGG_GLLWHLLHHLLH-NH2 | 7.8 |
| AMPM5 | K_SSSGGG_GLLWHLLHHLLH-NH2 | 15.6 |
| MA-AMPM1 | MA-K_GGGSG_GLLWHLLHHLLH (COOH) | 125 |
| MA-AMPM3 | MA-K_GGG_GLLWHLLHHLLH-NH2 | 7.8 |
| MA-AMPM5 | MA_K_SSSGGG_GLLWHLLHHLLH-NH2 | 15.6 |
| AMP2-NH2 | KWKRWWWWR-NH2 | 3.9 |
| AMPM7 | K_GGG_KWKRWWWWR-NH2 | 7.8 |
| AMPM8 | K_SSSGGG_KWKRWWWWR-NH2 | 31.3 |
| MA-AMPM7 | MA-K_GGG_KWKRWWWWR-NH2 | 7.8 |
| MA-AMPM8 | MA-K_SSSGGG_KWKRWWWWR-NH2 | 62.5 |
| MMES-AMPM7 | MMES-K_GGG_KWKRWWWWR-NH2 | 7.8 |
Fig. 4.
(a) The viability of S. mutans cultures after overnight incubation with polymerized discs containing non-AMP-monomer or AMP-monomers. S. mutans: a positive control without a disc. Peptide control: MA-nonAMP with GGG as a spacer. MA-C0 is the methacrylate control polymer replacing methacrylate peptide conjugates with methacrylate monomer, and (b) the viability of S. mutans cultures after overnight incubation with polymerized discs containing MA-AMPM7 or MMES-AMPM7 monomers. S. mutans: a positive control without a disc.
Computationally generated secondary-structure ensembles were used to estimate the changes in secondary structure of the active antimicrobial peptide domain with the selected spacer sequences. As a description of the patterns of hydrogen bonds in the peptide backbone, the secondary-structure ensembles have been shown to model conformations of the peptide that are associated with increasing concentrations of the kosmotropic agent tetrafluoroethanol (TFE). These structures may be more informative of folding behavior when the peptide acts in more ordered environments, such as in the bacterial membrane. Hydrogen bond patterns can be used as an indication on the effect of the spacer sequence if the twisting motion of the spacer is significantly different than the motion occurring in the free peptide. This twisting motion propensity is estimated by computationally folding the peptide with the spacer and comparing the hydrogen bonding patterns seen in the models with varying spacers and without a spacer. Based on these analyses, the GGG spacer has produced less secondary structural feature shifts than the SSSGGG spacer. Minimizing conformational shift may be one way to display improved functionality of the active domain. Our current models incorporate the secondary structure predicted in solution as an indirect method for estimated antimicrobial activity in solution as well as an integral part of the adhesive system.
In solution activity of a modified peptide can be different than its conjugated activity within a polymer network. Interestingly, the MIC values of MA-AMPM7 and MMES-AMPM7 in the monomeric state were the same (Table 1). When the antimicrobial activity of AMP-polymer conjugates against S. mutans was investigated, MA-AMPM7 did not display significant inhibition against S. mutans (Fig. 4). However, MMES-AMPM7 significantly improved antimicrobial activity as compared to MA-AMPM7 in the same crosslinked conjugates (Fig. 4b). The improved antimicrobial activity following polymerization associated with the monomer MMES may be attributed to the conformational differences of the secondary structure of the active antimicrobial peptide domain based upon the length of monomer MA and MMES.
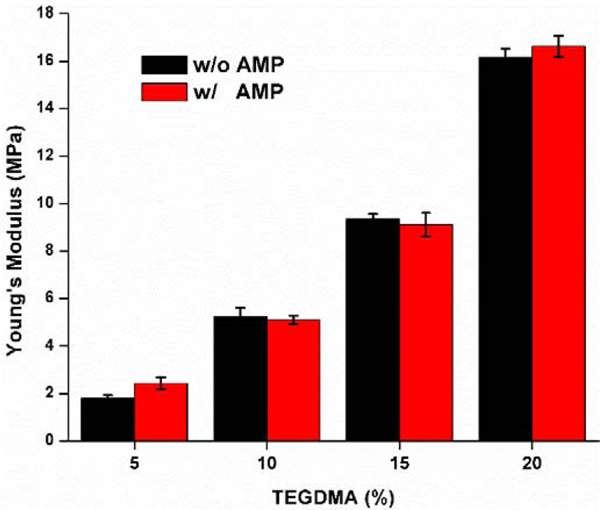
We tested the mechanical properties of engineered AMP-polymer conjugates using compression testing. With the same crosslinker (TEGDMA) concentration, the Young’s moduli of the control and experimental (w/o peptide) specimens are found to be comparable at the level of 0.05 (Fig. 5). As the TEGDMA increased from 5, 10, 15, to 20 wt%, the Young’s moduli of the control samples were recorded as 1.81±0.13, 5.23±0.38, 9.36±0.20, and 16.17±0.35 MPa, respectively. Meanwhile, the corresponding AMP-polymer conjugates resulted in Young’s moduli of 2.43±0.26, 5.10±0.18, 9.12±0.49, and 16.63±0.45 MPa, respectively, which are comparable to the modulus of the formulations along the water-adhesive phase boundary94–95. The addition of AMP monomers in the formulation does not indicate a loss of stiffness of the tested adhesive materials. AMP-hydrogels have been reported to achieve superior antimicrobial efficacy, however their mechanical strength was reported to be compromised limiting their potential application in dental restorative materials70–71, 76, 78–79, 81–83. Engineered AMP-polymer conjugates offers promising path to further explore as alternative restorative constructs maintaining the mechanical strength while providing antimicrobial properties.
Fig. 5.
Young’s moduli of the controls and AMP-polymer conjugates cylindrical samples.
4. Hybrid to biohybrid design: the strategy to achieve superior mechanical performance
During acid etching, the mineral phase of dentin is removed to expose the collagen and with the wet bonding technique, the collagen matrix remains moist to avoid collapse. Adhesive infiltrates the wet collagen matrix, the adhesive undergoes in situ polymerization and the collagen-polymerized adhesive construct, i.e. the hybrid layer, is formed. As noted above (sections 1 and 2), the mechanical properties of the hybrid layer deteriorate under aging conditions relevant to in vivo function.
Demineralization of dentin is one of the major reason for the deterioration of mechanical properties. There have been different top-down and bottom-up approaches proposed to address this problem15, 96–97. The lack of seed crystallites in the hybrid layer will significantly limit the remineralization, epitaxial growth over the seed crystallites as a classical top down approach was used to address this problem. Whereas bottom up approach involves protein, peptides, or biomimetic analogs to mediate or template the nucleation and growth within the collagen matrix 97. Peptide mediated remineralization of the dentin can be one approach to mitigate the negative impact of the deteriorating mechanical properties. Nevertheless, controlling the orientation of crystallites and achieving complete remineralization still present a challenge15, 98. Indeed, atomic computations suggest complex binding interactions between peptides and mineral surfaces that prefer specific conformations and compatible crystallite habit99.
Along with the remineralization approach, our polymer chemistry investigations have led us to new strategies for addressing deteriorating mechanical properties that are generally noted in dental adhesives under in vivo conditions. We reported the self-strengthening strategy to reinforce the polymethacrylate-based dental adhesive by introducing photoacid-induced sol-gel reaction45, 100–102 for the first time. The results indicated that in both neutral and acidic conditions, the self-strengthening significantly improve the mechanical properties. By tuning the alkoxysilane monomer’s structure and functionalities, we have engineered dental adhesives that provide the requisite hydrophilic-hydrophobic balance, a more homogenous structure and self-strengthening properties 100, 102.
4.1. Peptide Mediated Remineralization
Endogenous enzymes such as matrix metalloproteinases that degrade the exposed demineralized dentin; bacteria and saliva enzymes and factors such as chemical and enzymatic hydrolysis cause deterioration of the bond between dentin and low viscosity adhesive used to connect the composite to the tooth103. In the process following this degradation, the bacteria penetrate the interface, the cariogenic plaque accumulates in the exposed, demineralized dentin leading ultimately to decay and failure of the composite restoration. Failure to maintain the integrity of the a/d bond reduces the clinical lifetime of composite restorations 104–105. Remineralization of deficient/damaged dentin matrices at the a/d interface, mediated by peptides, 106–107 provides a viable solution to this problem.
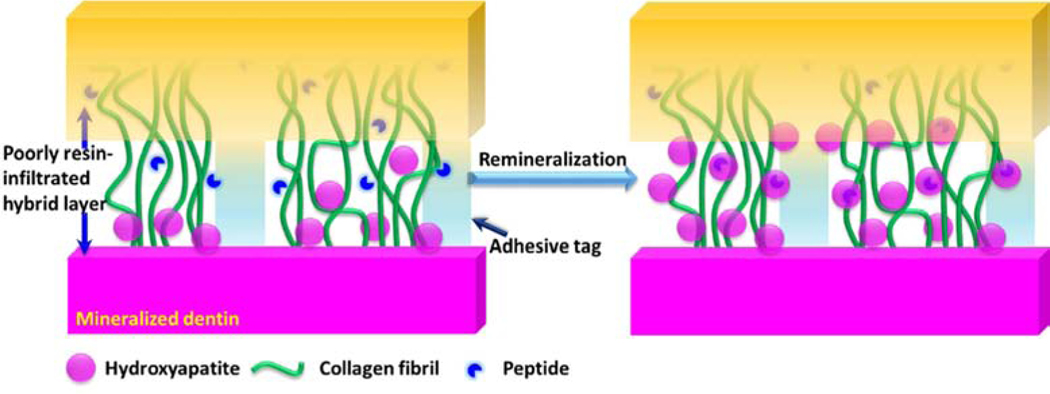
To mimic biomolecular interactions at the material-tissue interface, we as well as many other groups selected peptides for metals, minerals and semiconductors using combinatorial biology protocols, e.g., phage and cell surface displays108–122. We utilized these peptides in several bioactive and antimicrobial surface design, inorganic material synthesis and directed nanoparticle or biomolecule assembly121, 123–134,135. Our prior art includes a hydroxyapatite binding peptide (HABP: CMLPHHGAC) selected using phage display method demonstrating a control over the hydroxyapatite mineralization kinetics and resulting in a specific morphology136. In a different study this peptide was also shown to bind to the mineralized tissues after incorporating into a fluorescent probe 137. We further explored this peptide for achieving remineralization at the biohybrid layer (Fig. 6)
Fig. 6.
Biohybrid layer achieved through peptide mediated remineralization.
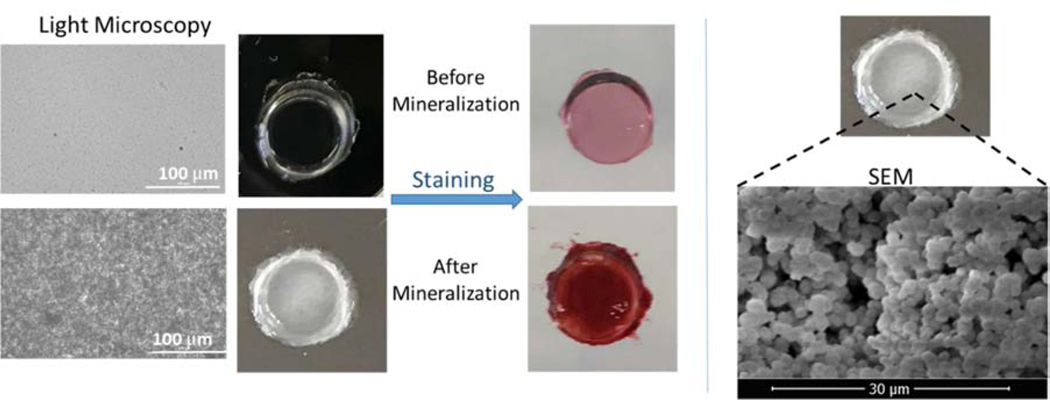
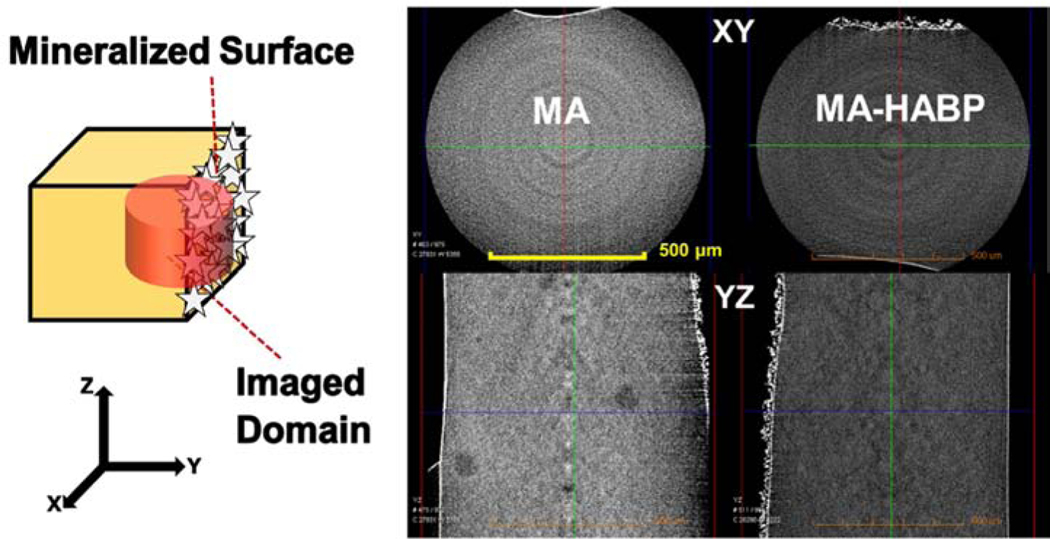
Remineralization is one clear component of a multi-faceted strategy to achieve a durable, integrated a/d interface that will provide a critical barrier between the repaired tooth and the oral environment. The HABP peptide that is genetically inserted into a green fluorescent protein was explored to mineralize deficient dentin matrices at the a/d interface. Our analyses on the collagen, adhesive and mineral demonstrated the homogenous distribution of mineral achieved throughout the dentin interface. 138. We next designed an engineered peptide-based copolymer system using a spacer integrated HABP derivative to conjugate it to methacrylate. When resulting methacrylate-HABP monomers were copolymerized into dental adhesive formulation, we observed a mineral forming effect using an alizarin red staining assay (Fig. 7). Moreover, the spherical particle formed was confirmed by the SEM images (Fig. 7). The overall porosity of the dental adhesive structure decreases in peptide-polymer conjugates and mineralization favors high porosity. Our results demonstrates that significant mineralization is achieved in the polymer-peptide conjugate structure as compared to the polymer only structure (Fig. 8).
Fig. 7.
Light microscopy and SEM images of peptide mediated mineralization on polymer discs. Peptide integrated polymer discs were stained with Alizarin Red.
Fig. 8.
Micro-CT images of mineralization with and without peptide mediation on polymer disks.
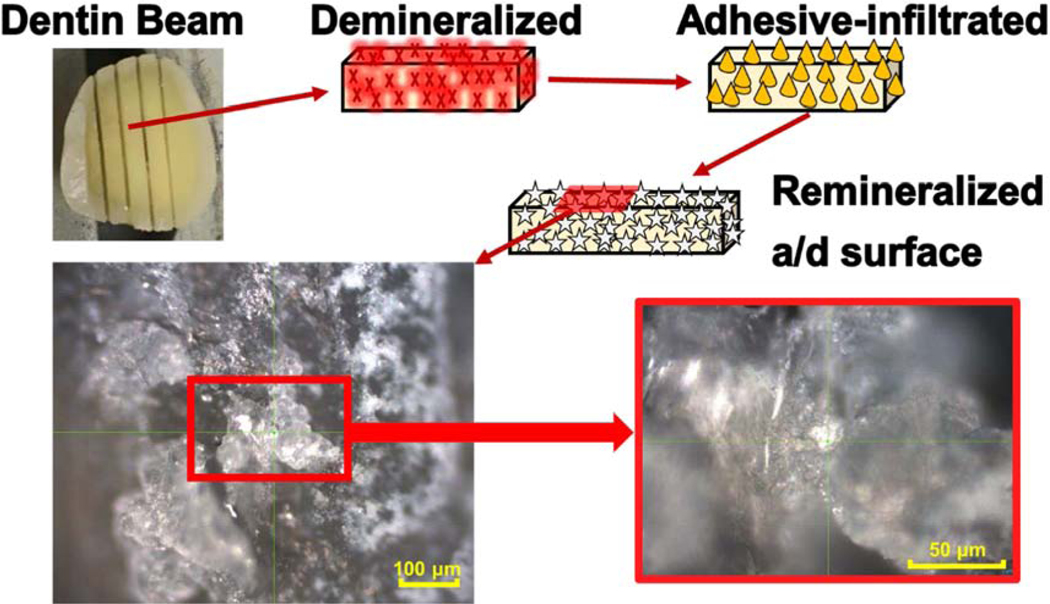
Building on these promising results, we studied the potential of using peptide-protein conjugates to achieve peptide-mediated mineralization at the a/d interface. Fig. 9 reveals that significant remineralization is achieved at the a/d interface, which was exposed to mineralization solution after being demineralized and then infiltrated with adhesive that contains peptide. Further investigation is required to optimize the peptide-tethered-adhesive to provide peptide-mediated mineralization under conditions relevant to the in vivo settings.
Fig. 9.
Light microscopy images showing peptide-mediated mineralization at a/d interface.
4.2. New polymer chemistries: self-strengthening property
In our previous investigations,45, 100–102 the self-strengthening adhesives showed: 1) formulation with lower viscosity and higher C=C bond conversion; 2) significantly improved crosslinking density and mechanical performance in wet conditions; 3) dramatically reduced leachates, especially HEMA. These results support the use of self-strengthening adhesives as one component of a multi-faceted strategy to promote the durability and integrity of the a/d interface.
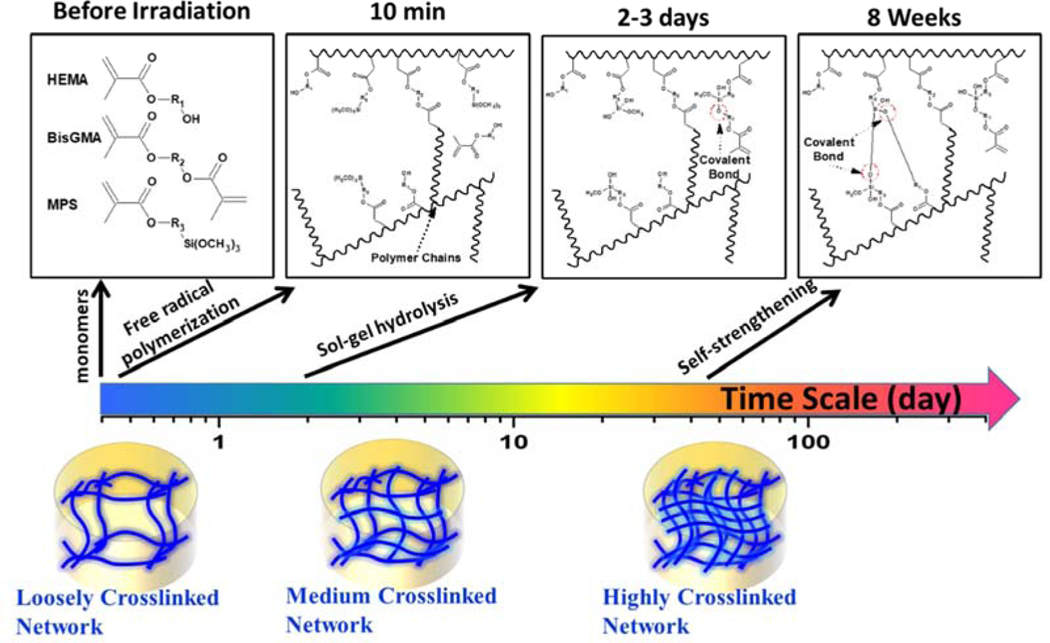
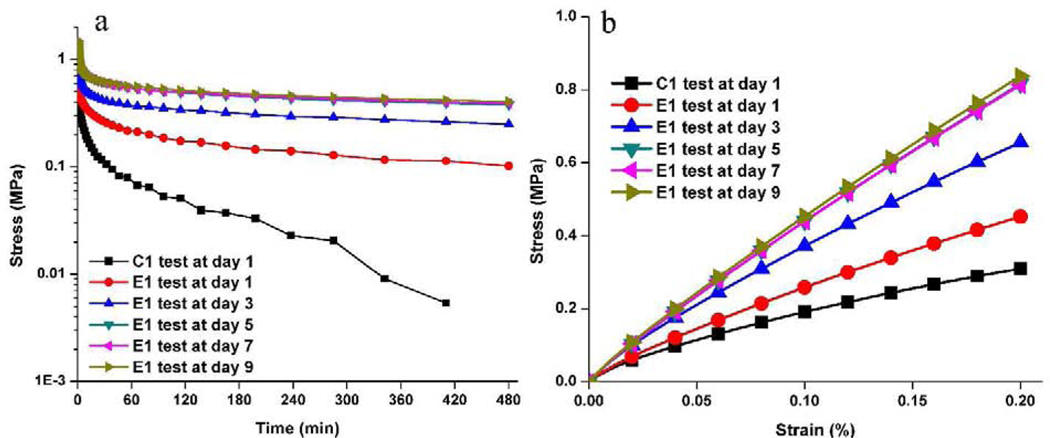
The novel alkoxysilane-containing adhesives capitalize on free-radical polymerization and sol-gel reactions to provide self-strengthening polymers. The relatively slow rate of the photoacid-induced sol-gel reaction compared to the free-radical polymerization provides a novel way to tune the network structure after light irradiation. Fig. 10 shows the proposed mechanism for the self-strengthening reactions after light irradiation. When the liquid resin is irradiated by visible-light, the polymethacrylate-based matrix is formed by free radical polymerization of the co-monomers, e.g., HEMA and BisGMA. Simultaneously, the alkoxysilane groups are hydrolyzed in a reaction catalyzed by the photoacid produced during the visible-light irradiation. Soaking the resin in water or lactic acid furthers the autonomous hydrolysis and condensation of the alkoxysilyl moieties, creating new crosslink points. The resulting silanol groups react with the hydroxyl groups of HEMA or BisGMA to form covalent bonds. The autonomic sol-gel reaction continues in the wet environment, leading to intrinsic reinforcement of the network. As Fig. 11 indicates, the simulation based on Prony series-fitting139 showed that the mechanical properties of polymers were significantly improved by the self-strengthening reaction in the first few days whereas the formulation without self-strengthening property fails after the first testing day (Sarikaya et al., unpublished observations). With the increase in soaking time, the effect of self-strengthening reaction on the stress relaxation behavior of bulk polymer decreases due to the gradual increase in the crosslinking density of the polymer. In comparison, the DMA test results indicated that the self-strengthening contributed to the further crosslinking reaction even after 8 weeks soaking in water45, 102. Within the polymethacrylate-based matrix, the self-strengthening adhesive provided a slow, persistent crosslinking reaction, which can promote the formation of Si-O-Si bonds and improve the hydrolytic resistance.
Fig. 10.
The evolution of network structure in adhesive through self-strengthening reaction.
Fig. 11.
Improved mechanical performance of the self-strengthening polymer adhesive over time: (a) Stress-relaxation test results and (b) Stress-strain behavior prediction based on Prony-series-fitted stress-relaxation-test data. Stress relaxation test informs that HEMA/BISGMA/MPS formulation (E1) is superior to HEMA/BISGMA/MES formulation (C1) in terms of stiffening behavior in wet conditions.
6. Conclusions
The bioinspired materials design approach offers significant promise for promoting the integrity of the a/d interface and providing a concomitant increase in the lifetime of composite restorations. Here we summarize synergistically working bio-hybrid constructs that are designed by engineering peptides combined with new polymer chemistries. Discrete peptides are tethered to polymers to provide multi-bio-functional adhesive formulations that simultaneously achieve antimicrobial and remineralization properties. Spacer sequences are used to provide reactive groups for simultaneously tethering functionally distinctive peptides to the monomer as well as providing the length and flexibility required to maintain the peptide’s original bioactivities when applied as an adhesive at the tooth surface. Examples on peptide-polymer conjugates are provided to remineralize the deficient/mineral-depleted dentin matrices as well as to promote antimicrobial activity at the a/d interface without compromising the polymer`s mechanical properties. This hybrid approach includes new polymer chemistries resulting dental adhesives with self-strengthening properties, enhanced hydrolytic stability and decreased degradant release.
The inherent specificity of peptides makes them ideal molecules for conjugation with synthetic polymers to create new functional biomaterials. The physical limitations of peptides, such as their sensitivity to pH, temperature, and degradation, could be mitigated through conjugation with polymers34–35. However, conjugation often leads to a significant reduction in the peptide’s bioactivity. Typically, the polymer-peptide conjugates are soft materials that are used in solution, hydrogels, or loosely crosslinking gels to maintain the peptide’s bioactivity35, 43. Therefore, one important consideration for the peptide-polymer conjugate used in a dental adhesive application is the balance between bioactivity, durability and mechanical properties. To address this challenge, optimization of the peptide bioactivity and control of chemical conditions such as the ratio of peptide-to-polymer, as well as the resin components, should be thoroughly investigated. With the fundamental knowledge of structural and biological properties of polymer-peptide conjugates, the investigations can lead to an ability to tailor the conjugates to meet the needs of specific applications. The efforts to develop polymer-peptide conjugates have the potential of providing new roadmaps for material design and this work offers promise for advancing the development of materials for a variety of biomedical applications 42, 140–141. The resulting biofunctional materials may be exploited in a wide variety of applications including, but not limited to, the treatment of secondary caries, enhanced durability of dental composite restorations, antimicrobial gels, and tissue engineering applications. In tissue engineering, free-radical crosslinked polymers are desirable because they can be polymerized in direct contact with tissues, either in solution, or in thin layer on the surface141–143. Polymer-peptide conjugates hold great promise as a new class of hybrid biomaterials with diverse attributes, such as antimicrobial properties,144–145 cell-penetration scaffolds,146 mineralized tissue repair and bioadhesive properties147. With the introduction of self-strengthening characteristic, the conjugates can be tailored to achieve target properties required for specific biomedical applications. This strategy could be introduced into the biomaterials to tune the mechanical properties, e.g. viscoelasticity, which is desirable for tissue engineering, and numerous other applications, such as 3D printing, wound dressing, scaffold materials, as well as bone regeneration.
Acknowledgements
This work was supported by research grant R01DE025476 (PS, CT) from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Maryland and the University of Kansas Research GO initiative (PS).
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pashley DH; Tay FR; Breschi L; Tjaderhane L; Carvalho RM; Carrilho M; Tezvergil-Mutluay A, State of the art etch-and-rinse adhesives. Dent. Mater 2011, 27 (1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalesinskas P; Kacergius T; Ambrozaitis A; Peciuliene V; Ericson D, Reducing dental plaque formation and caries development. A review of current methods and implications for novel pharmaceuticals. Stomatologija 2014, 16 (2), 44–52. [PubMed] [Google Scholar]
- 3.Tjaderhane L; Nascimento FD; Breschi L; Mazzoni A; Tersariol ILS; Geraldeli S; Tezvergil-Mutluay A; Carrilho M; Carvalho RM; Tay FR; Pashley DH, Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent. Mater 2013, 29 (10), 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breschi L; Mazzoni A; Ruggeri A; Cadenaro M; Di Lenarda R; Dorigo ED, Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater 2008, 24 (1), 90–101. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RS; Fransman R, Adhesive dentistry and endodontics: Materials, clinical strategies and procedures for restoration of access cavities: A review. J. Endod 2005, 31 (3), 151–165. [DOI] [PubMed] [Google Scholar]
- 6.De Munck J; Van Landuyt K; Peumans M; Poitevin A; Lambrechts P; Braem M; Van Meerbeek B, A critical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res 2005, 84 (2), 118–132. [DOI] [PubMed] [Google Scholar]
- 7.Collaborators GBDOD; Bernabe E; Marcenes W; Hernandez CR; Bailey J; Abreu LG; Alipour V; Amini S; Arabloo J; Arefi Z; Arora A; Ayanore MA; Barnighausen TW; Bijani A; Cho DY; Chu DT; Crowe CS; Demoz GT; Demsie DG; Dibaji Forooshani ZS; Du M; El Tantawi M; Fischer F; Folayan MO; Futran ND; Geramo YCD; Haj-Mirzaian A; Hariyani N; Hasanzadeh A; Hassanipour S; Hay SI; Hole MK; Hostiuc S; Ilic MD; James SL; Kalhor R; Kemmer L; Keramati M; Khader YS; Kisa S; Kisa A; Koyanagi A; Lalloo R; Le Nguyen Q; London SD; Manohar ND; Massenburg BB; Mathur MR; Meles HG; Mestrovic T; Mohammadian-Hafshejani A; Mohammadpourhodki R; Mokdad AH; Morrison SD; Nazari J; Nguyen TH; Nguyen CT; Nixon MR; Olagunju TO; Pakshir K; Pathak M; Rabiee N; Rafiei A; Ramezanzadeh K; Rios-Blancas MJ; Roro EM; Sabour S; Samy AM; Sawhney M; Schwendicke F; Shaahmadi F; Shaikh MA; Stein C; Tovani-Palone MR; Tran BX; Unnikrishnan B; Vu GT; Vukovic A; Warouw TSS; Zaidi Z; Zhang ZJ; Kassebaum NJ, Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J Dent Res 2020, 99 (4), 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer P; Ye Q; Song LY; Parthasarathy R; Boone K; Misra A; Tamerler C, Threats to adhesive/dentin interfacial integrity and next generation bio-enabled multifunctional adhesives. J Biomed Mater Res B 2019, 107 (8), 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X; Huang X; Li M; Peng X; Wang S; Zhou X; Cheng L, Development and status of resin composite as dental restorative materials. Journal of Applied Polymer Science 2019, 136 (44), 48180. [Google Scholar]
- 10.Stewart CA; Finer Y, Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater 2019, 35 (1), 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opdam N; Frankenberger R; Magne P, From ‘Direct Versus Indirect’Toward an Integrated Restorative Concept in the Posterior Dentition. Operative dentistry 2016, 41 (S7), S27–S34. [DOI] [PubMed] [Google Scholar]
- 12.Nakabayashi N; Kojima K; Masuhara E, The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res 1982, 16 (3), 265–273. [DOI] [PubMed] [Google Scholar]
- 13.Sano H; Shono T; Takatsu T; Hosoda H, Microporous Dentin Zone beneath ResinImpregnated Layer. Operative Dentistry 1994, 19 (2), 59–64. [PubMed] [Google Scholar]
- 14.Bertassoni LE; Orgel JPR; Antipova O; Swain MV, The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater 2012, 8 (7), 2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y; Tjaderhane L; Breschi L; Mazzoni A; Li N; Mao J; Pashley DH; Tay FR, Limitations in Bonding to Dentin and Experimental Strategies to Prevent Bond Degradation. J Dent Res 2011, 90 (8), 953–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaidyanathan TK; Vaidyanathan J, Recent Advances in the Theory and Mechanism of Adhesive Resin Bonding to Dentin: A Critical Review. J Biomed Mater Res B 2009, 88B (2), 558–578. [DOI] [PubMed] [Google Scholar]
- 17.Spencer P; Swafford JR, Unprotected protein at the dentin-adhesive interface. Quintessence International 1999, 30 (7), 501–507. [PubMed] [Google Scholar]
- 18.Spencer P; Wang Y; Bohaty B, Interfacial chemistry of moisture-aged class II composite restorations. J Biomed Mater Res B 2006, 77B (2), 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer P; Ye Q; Park J; Topp EM; Misra A; Marangos O; Wang Y; Bohaty BS; Singh V; Sene F; Eslick J; Camarda K; Katz JL, Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng 2010, 38 (6), 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer P; Ye Q; Misra A; Goncalves SEP; Laurence JS, Proteins, Pathogens, and Failure at the Composite-Tooth Interface. J Dent Res 2014, 93 (12), 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari M; Tay FR, Technique sensitivity in bonding to vital, acid-etched dentin. Operative Dentistry 2003, 28 (1), 3–8. [PubMed] [Google Scholar]
- 22.Van Landuyt KL; Snauwaert J; De Munck J; Peurnans M; Yoshida Y; Poitevin A; Coutinho E; Suzuki K; Lambrechtsa P; Van Meerbeek B, Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28 (26), 3757–3785. [DOI] [PubMed] [Google Scholar]
- 23.Donmez N; Belli S; Pashley DH; Tay FR, Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res 2005, 84 (4), 355–359. [DOI] [PubMed] [Google Scholar]
- 24.Finer Y; Jaffer F; Santerre JP, Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials 2004. 25 (10), 1787–93. [DOI] [PubMed] [Google Scholar]
- 25.Finer Y; Santerre JP, The influence of resin chemistry on a dental composite’s biodegradation. J. Biomed. Mater. Res 2004, 69A, 233–46. [DOI] [PubMed] [Google Scholar]
- 26.Finer Y; Santerre JP, Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res 2004, 83, 22–6. [DOI] [PubMed] [Google Scholar]
- 27.Hagio M; Kawaguchi M; Motokawa W; Mizayaki K, Degradation of Methacrylate Monomers in Human Saliva. Dental Materials Journal 2006, 25 (2), 241–246. [DOI] [PubMed] [Google Scholar]
- 28.Munksgaard EC; Freund M, Enzymatic hydrolysis of (di)methacrylates and their polymers. Scandinavian Journal of Dental Research 1990, 98, 261–267. [DOI] [PubMed] [Google Scholar]
- 29.Yourtee DM; Smith RE; Russo KA; Burmaster S; Cannon JM; Eick JD; Kostoryz EL, The stability of methacrylate biomaterials when enzyme challenged: Kinetic and systematic evaluations. J. Biomed. Mater. Res 2001, 57 (4), 523–531. [DOI] [PubMed] [Google Scholar]
- 30.Bourbia M; Ma D; Cvitkovitch DG; Santerre JP; Finer Y, Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res 2013, 92 (11), 989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahyazadehfar M; Mutluay MM; Majd H; Ryou H; Arola D, Fatigue of the resin-enamel bonded interface and the mechanisms of failure. J. Mech. Behav. Biomed. Mater 2013, 21, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke FJT; Wilson NHF; Cheung SW; Mjor IA, Influence of patient factors on age of restorations at failure and reasons for their placement and replacement. J. Dent 2001, 29 (5), 317–324. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong SR; Keller JC; Boyer DB, Mode of failure in the dentin-adhesive resin-resin composite bonded joint as determined by strength-based (mu TBS) and fracture-based (CNSB) mechanical testing. Dent. Mater 2001, 17 (3), 201–210. [DOI] [PubMed] [Google Scholar]
- 34.Krishna OD; Kiick KL, Protein- and Peptide-Modified Synthetic Polymeric Biomaterials. Biopolymers 2010, 94 (1), 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shu JY; Panganiban B; Xu T, Peptide-Polymer Conjugates: From Fundamental Science to Application. Annu Rev Phys Chem 2013, 64, 631–657. [DOI] [PubMed] [Google Scholar]
- 36.Peptide-Polymer Conjugates Self-Assembly. Polym Advan Technol 2008, 19 (12), A3–A3. [Google Scholar]
- 37.Bas O; Hansske F; Lim J; Ravichandran A; Kemnitz E; Teoh SH; Hutmacher DW; Borner HG, Tuning mechanical reinforcement and bioactivity of 3D printed ternary nanocomposites by interfacial peptide-polymer conjugates. Biofabrication 2019, 11 (3). [DOI] [PubMed] [Google Scholar]
- 38.Dehn S; Chapman R; Jolliffe KA; Perrier S, Synthetic Strategies for the Design of Peptide/Polymer Conjugates. Polym Rev 2011, 51 (2), 214–234. [Google Scholar]
- 39.Kumar P; Takayesu A; Abbasi U; Kalathottukaren MT; Abbina S; Kizhakkedathu JN; Straus SK, Antimicrobial peptide–polymer conjugates with high activity: Influence of polymer molecular weight and peptide sequence on antimicrobial activity, proteolysis, and biocompatibility. ACS applied materials & interfaces 2017, 9 (43), 37575–37586. [DOI] [PubMed] [Google Scholar]
- 40.Line BR; Mitra A; Nan A; Ghandehari H, Targeting tumor angiogenesis: Ccomparison of peptide and polymer-peptide conjugates. J Nucl Med 2005, 46 (9), 1552–1560. [PubMed] [Google Scholar]
- 41.Luo TZ; Kiick KL, Collagen-like peptides and peptide-polymer conjugates in the design of assembled materials. Eur Polym J 2013, 49 (10), 2998–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitra A; Nan A; Papadimitriou JC; Ghandehari H; Line BR, Polymer-peptide conjugates for angiogenesis targeted tumor radiotherapy. Nucl Med Biol 2006, 33 (1), 43–52. [DOI] [PubMed] [Google Scholar]
- 43.Wilson P, Synthesis and Applications of Protein/Peptide‐Polymer Conjugates. Macromolecular Chemistry and Physics 2017, 218 (9), 1600595. [Google Scholar]
- 44.Webber MJ; Appel EA; Meijer EW; Langer R, Supramolecular biomaterials. Nat Mater 2016, 15 (1), 13–26. [DOI] [PubMed] [Google Scholar]
- 45.Song LY; Ye Q; Ge XP; Misra A; spencer P, Mimicking Nature: Self-strengthening Properties in a Dental Adhesive Acta Biomater 2016, 35, 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho RM; Manso AP; Geraldeli S; Tay FR; Pashley DH, Durability of bonds and clinical success of adhesive restorations. Dent. Mater 2012, 28 (1), 72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perdigao J, Dentin bonding-Variables related to the clinical situation and the substrate treatment. Dent. Mater 2010, 26 (2), E24–E37. [DOI] [PubMed] [Google Scholar]
- 48.Frassetto A; Breschi L; Turco G; Marchesi G; Di Lenarda R; Tay FR; Pashley DH; Cadenaro M, Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability-A literature review. Dent. Mater 2016, 32 (2), E41–E53. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto M; Fujita S; Endo K; Ohno H, Effect of dentinal water on bonding of self-etching adhesives. Dental Materials Journal 2009, 28 (5), 634–641. [DOI] [PubMed] [Google Scholar]
- 50.Sano H; Takatsu T; Ciucchi B; Russell CM; Pashley DH, Tensile Properties of Resin-Infiltrated Demineralized Human Dentin. J Dent Res 1995, 74 (4), 1093–1102. [DOI] [PubMed] [Google Scholar]
- 51.Pashley DH; Agee KA; Wataha JC; Rueggeberg F; Ceballos L; Itou K; Yoshiyama M; Carvalho RM; Tay FR, Viscoelastic properties of demineralized dentin matrix. Dent. Mater 2003, 19 (8), 700–706. [DOI] [PubMed] [Google Scholar]
- 52.Wang G; Li X; Wang Z, APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 2016, 44 (D1), D1087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X; Wu H; Lu H; Li G; Huang Q, LAMP: A database linking antimicrobial peptides. PLoS One 2013, 8 (6), e66557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobson A; O’connor P; Cotter P; Ross R; Hill C, Impact of the broad‐spectrum antimicrobial peptide, lacticin 3147, on Streptococcus mutans growing in a biofilm and in human saliva. Journal of applied microbiology 2011, 111 (6), 1515–1523. [DOI] [PubMed] [Google Scholar]
- 55.Wang W; Tao R; Tong Z; Ding Y; Kuang R; Zhai S; Liu J; Ni L, Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides 2012, 33 (2), 212–219. [DOI] [PubMed] [Google Scholar]
- 56.Li H; Cheng JW; Yu HY; Xin Y; Tang L; Ma Y, Effect of the antimicrobial peptide D-Nal-Pac-525 on the growth of Streptococcus mutans and its biofilm formation. Journal of microbiology and biotechnology 2013, 23 (8), 1070–1075. [DOI] [PubMed] [Google Scholar]
- 57.Shang D; Liang H; Wei S; Yan X; Yang Q; Sun Y, Effects of antimicrobial peptide L-K6, a temporin-1CEb analog on oral pathogen growth, Streptococcus mutans biofilm formation, and anti-inflammatory activity. Applied microbiology and biotechnology 2014, 98 (20), 8685–8695. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Gomez S; Martinez-de-Tejada G, Antimicrobial peptides as anti-biofilm agents in medical implants. Current topics in medicinal chemistry 2017, 17 (5), 590–603. [DOI] [PubMed] [Google Scholar]
- 59.Bagheri M; Arasteh S; Haney EF; Hancock RE, Tryptic stability of synthetic bactenecin derivatives is determined by the side chain length of cationic residues and the peptide conformation. J Med Chem 2016, 59 (7), 3079–86. [DOI] [PubMed] [Google Scholar]
- 60.Belanger CR; Mansour SC; Pletzer D; Hancock REW, Alternative strategies for the study and treatment of clinical bacterial biofilms. Emerging Topics in Life Sciences 2017. [DOI] [PubMed] [Google Scholar]
- 61.Cherkasov A; Hilpert K; Jenssen H; Fjell CD; Waldbrook M; Mullaly SC; Volkmer R; Hancock RE, Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem Biol 2009, 4 (1), 65–74. [DOI] [PubMed] [Google Scholar]
- 62.Yazici H; O’Neill MB; Kacar T; Wilson BR; Oren EE; Sarikaya M; Tamerler C, Engineered chimeric peptides as antimicrobial surface coating agents toward infection-free implants. ACS applied materials & interfaces 2016, 8 (8), 5070–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holmberg KV; Abdolhosseini M; Li Y; Chen X; Gorr SU; Aparicio C, Bioinspired stable antimicrobial peptide coatings for dental applications. Acta Biomater 2013, 9 (9), 8224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aida KL; Kreling PF; Caiaffa KS; Calixto GMF; Chorilli M; Spolidorio DMP; Santos NA; Cilli EM; Duque C, Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. International Journal of Nanomedicine 2018, 13, 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie S-X; Boone K; VanOosten SK; Yuca E; Song L; Ge X; Ye Q; Spencer P; Tamerler C, Peptide Mediated Antimicrobial Dental Adhesive System. Applied Sciences 2019, 9 (3), 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mai S; Mauger MT; Niu LN; Barnes JB; Kao S; Bergeron BE; Ling JQ; Tay FR, Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater 2017, 49, 16–35. [DOI] [PubMed] [Google Scholar]
- 67.Su M; Yao S; Gu L; Huang Z; Mai S, Antibacterial effect and bond strength of a modified dental adhesive containing the peptide nisin. Peptides 2018, 99, 189–194. [DOI] [PubMed] [Google Scholar]
- 68.de Arauz LJ; Jozala AF; Mazzola PG; Penna TCV, Nisin biotechnological production and application: a review. Trends in Food Science & Technology 2009, 20 (3–4), 146–154. [Google Scholar]
- 69.Heredia KL; Maynard HD, Synthesis of protein–polymer conjugates. Organic & biomolecular chemistry 2006, 5 (1), 45–53. [DOI] [PubMed] [Google Scholar]
- 70.Sun H; Hong Y; Xi Y; Zou Y; Gao J; Du J, Synthesis, self-assembly, and biomedical applications of antimicrobial peptide–polymer conjugates. Biomacromolecules 2018, 19 (6), 1701–1720. [DOI] [PubMed] [Google Scholar]
- 71.González-Henríquez C; Sarabia-Vallejos M; Rodriguez-Hernandez J, Advances in the fabrication of antimicrobial hydrogels for biomedical applications. Materials 2017, 10 (3), 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malmsten M, Antimicrobial and antiviral hydrogels. Soft Matter 2011, 7 (19), 8725–8736. [Google Scholar]
- 73.Babavalian H; Latifi AM; Shokrgozar MA; Bonakdar S; Mohammadi S; Moghaddam MM, Analysis of healing effect of alginate sulfate hydrogel dressing containing antimicrobial peptide on wound infection caused by methicillin-resistant Staphylococcus aureus. Jundishapur journal of microbiology 2015, 8 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva J; Dhall S; Chan A; Gama M; Mgreen M, improved Burn Wound Healing By An Antimicrobial Peptide Released From Conjugates With Dextrin Embedded In A Carbopol Hydrogel. Wound Repair and Regeneration 2015, 23 (2), A40. [DOI] [PubMed] [Google Scholar]
- 75.Xie Z; Aphale NV; Kadapure TD; Wadajkar AS; Orr S; Gyawali D; Qian G; Nguyen KT; Yang J, Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing. Journal of Biomedical Materials Research Part A 2015, 103 (12), 3907–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang R; Xu D.-l.; Liang L; Xu T.-t.; Liu W; Ouyang P.-k.; Chi B; Xu H, Enzymatically crosslinked epsilon-poly-L-lysine hydrogels with inherent antibacterial properties for wound infection prevention. RSC Advances 2016, 6 (11), 8620–8627. [Google Scholar]
- 77.Buffet-Bataillon S; Tattevin P; Bonnaure-Mallet M; Jolivet-Gougeon A, Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. International journal of antimicrobial agents 2012, 39 (5), 381–389. [DOI] [PubMed] [Google Scholar]
- 78.Nichol JW; Koshy ST; Bae H; Hwang CM; Yamanlar S; Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31 (21), 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Annabi N; Rana D; Sani ES; Portillo-Lara R; Gifford JL; Fares MM; Mithieux SM; Weiss AS, Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yi X; He J; Wang X; Zhang Y; Tan G; Zhou Z; Chen J; Chen D; Wang R; Tian W, Tunable mechanical, antibacterial, and cytocompatible hydrogels based on a functionalized dual network of metal coordination bonds and covalent crosslinking. ACS applied materials & interfaces 2018, 10 (7), 6190–6198. [DOI] [PubMed] [Google Scholar]
- 81.Song A; Rane AA; Christman KL, Antibacterial and cell-adhesive polypeptide and poly (ethylene glycol) hydrogel as a potential scaffold for wound healing. Acta Biomater 2012, 8 (1), 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavel PK; Dev D; Parmar HS; Bhasin S; Das AK, Investigations of Peptide-Based Biocompatible Injectable Shape-Memory Hydrogels: Differential Biological Effects on Bacterial and Human Blood Cells. ACS applied materials & interfaces 2018, 10 (13), 10729–10740. [DOI] [PubMed] [Google Scholar]
- 83.Shams Es-haghi S; Mayfield MB; Weiss R, Effect of Freeze/Thaw Process on Mechanical Behavior of Double-Network Hydrogels in Finite Tensile Deformation. Macromolecules 2018, 51 (3), 1052–1057. [Google Scholar]
- 84.Papadogiannis D; Lakes RS; Papadogiannis Y; Tolidis K, Mechanical viscoelastic behavior of dental adhesives. Dental materials : official publication of the Academy of Dental Materials 2013, 29 (6), 693–701. [DOI] [PubMed] [Google Scholar]
- 85.Hosaka K; Nakajima M; Takahashi M; Itoh S; Ikeda M; Tagami J; Pashley DH, Relationship between mechanical properties of one-step self-etch adhesives and water sorption. Dental materials : official publication of the Academy of Dental Materials 2010, 26 (4), 360–7. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y; Fan Y; Zhou Z; Tu H; Ren Q; Wang X; Ding L; Zhou X; Zhang L, De novo synthetic short antimicrobial peptides against cariogenic bacteria. Archives of oral biology 2017, 80, 41–50. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y; Wang X; Jiang W; Wang K; Luo J; Li W; Zhou X; Zhang L, Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. Journal of oral microbiology 2018, 10 (1), 1442089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y; Zeng Y; Wang Y; Li H; Yu S; Jiang W; Li Y; Zhang L, Antimicrobial peptide GH12 targets Streptococcus mutans to arrest caries development in rats. Journal of Oral Microbiology 2019, 11 (1), 1549921. [Google Scholar]
- 89.Yucesoy DT; Hnilova M; Boone K; Arnold PM; Snead ML; Tamerler C, Chimeric peptides as implant functionalization agents for titanium alloy implants with antimicrobial properties. JOM 2015, 67 (4), 754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costa F; Carvalho IF; Montelaro RC; Gomes P; Martins MCL, Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater 2011, 7 (4), 1431–1440. [DOI] [PubMed] [Google Scholar]
- 91.Wisdom C; VanOosten SK; Boone KW; Khvostenko D; Arnold PM; Snead ML; Tamerler C, Controlling the Biomimetic Implant Interface: Modulating Antimicrobial Activity by Spacer Design. Journal of molecular and engineering materials 2016, 4 (01), 1640005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sengupta A; Thai CK; Sastry M; Matthaei JF; Schwartz DT; Davis EJ; Baneyx F, A genetic approach for controlling the binding and orientation of proteins on nanoparticles. Langmuir 2008, 24 (5), 2000–2008. [DOI] [PubMed] [Google Scholar]
- 93.Sedlak RH; Hnilova M; Grosh C; Fong H; Baneyx F; Schwartz D; Sarikaya M; Tamerler C; Traxler B, An engineered Escherichia coli silver-binding periplasmic protein promotes silver tolerance. Applied and environmental microbiology 2012, AEM. 06823–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Misra A; Parthasarathy R; Ye Q; Singh V; Spencer P, Swelling equilibrium of dentin adhesive polymers formed on the water–adhesive phase boundary: experiments and micromechanical model. Acta Biomater 2014, 10 (1), 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parthasarathy R; Misra A; Song L; Ye Q; Spencer P, Structure–property relationships for wet dentin adhesive polymers. Biointerphases 2018, 13 (6), 061004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong TS; Brough B; Ho CM, Creation of functional micro/nano systems through top-down and bottom-up approaches. Mol Cell Biomech 2009, 6 (1), 1–55. [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y; Mai S; Li N; Yiu CKY; Mao J; Pashley DH; Tay FR, Differences between top-down and bottom-up approaches in mineralizing thick, partially demineralized collagen scaffolds. Acta Biomater 2011, 7 (4), 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwendicke F; Al-Abdi A; Moscardo AP; Cascales AF; Sauro S, Remineralization effects of conventional and experimental ion-releasing materials in chemically or bacterially-induced dentin caries lesions. Dent. Mater 2019, 35 (5), 772–779. [DOI] [PubMed] [Google Scholar]
- 99.Poudel L; Tamerler C; Misra A; Ching W-Y, Atomic-scale quantification of interfacial binding between peptides and inorganic crystals: The case of calcium carbonate binding peptide on aragonite. The Journal of Physical Chemistry C 2017, 121 (51), 28354–28363. [Google Scholar]
- 100.Song LY; Ye Q; Ge XP; Misra A; Tamerler C; Spencer P, New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives. Acta Biomater 2019, 83, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song LY; Ye Q; Ge XP; Misra A; Tamerler C; Spencer P, Fabrication of hybrid crosslinked network with buffering capabilities and autonomous strengthening characteristics for dental adhesives. Acta Biomater 2018, 67, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song LY; Ye Q; Ge XP; Misra A; Tamerler C; Spencer P, Self-strengthening hybrid dental adhesive via visible-light irradiation triple polymerization. Rsc Advances 2016, 6 (57), 52434–52447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferracane JL; Hilton TJ, Polymerization stress--is it clinically meaningful? Dental materials : official publication of the Academy of Dental Materials 2016, 32 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- 104.Spencer P; Ye Q; Park J; Topp EM; Misra A; Marangos O; Wang Y; Bohaty BS; Singh V; Sene F; Eslick J; Camarda K; Katz JL, Adhesive/Dentin interface: the weak link in the composite restoration. Annals of biomedical engineering 2010, 38 (6), 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Opdam NJ; Bronkhorst EM; Loomans BA; Huysmans MC, 12-year survival of composite vs. amalgam restorations. J Dent Res 2010, 89 (10), 1063–7. [DOI] [PubMed] [Google Scholar]
- 106.Kirkham J; Firth A; Vernals D; Boden N; Robinson C; Shore RC; Brookes SJ; Aggeli A, Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res 2007, 86 (5), 426–30. [DOI] [PubMed] [Google Scholar]
- 107.Li Q-L; Ning T-Y; Cao Y; Zhang W.-b.; Mei ML; Chu CH, A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnology 2014, 14 (1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baneyx F; Schwartz DT, Selection and analysis of solid-binding peptides. Current opinion in biotechnology 2007, 18 (4), 312–317. [DOI] [PubMed] [Google Scholar]
- 109.Care A; Bergquist PL; Sunna A, Solid-binding peptides: smart tools for nanobiotechnology. Trends in biotechnology 2015, 33 (5), 259–268. [DOI] [PubMed] [Google Scholar]
- 110.Chiu CY; Li Y; Ruan L; Ye X; Murray CB; Huang Y, Platinum nanocrystals selectively shaped using facet-specific peptide sequences. Nature chemistry 2011, 3 (5), 393–9. [DOI] [PubMed] [Google Scholar]
- 111.Donatan S; Yazici H; Bermek H; Sarikaya M; Tamerler C; Urgen M, Physical elution in phage display selection of inorganic-binding peptides. Materials Science and Engineering: C 2009, 29 (1), 14–19. [Google Scholar]
- 112.Evans JS; Samudrala R; Walsh TR; Oren EE; Tamerler C, Molecular design of inorganic-binding polypeptides. Mrs Bulletin 2008, 33 (5), 514–518. [Google Scholar]
- 113.Gaskin DJ; Starck K; Vulfson EN, Identification of inorganic crystal-specific sequences using phage display combinatorial library of short peptides: a feasibility study. Biotechnology letters 2000, 22 (15), 1211–1216. [Google Scholar]
- 114.Hayashi T; Sano K-I; Shiba K; Kumashiro Y; Iwahori K; Yamashita I; Hara M, Mechanism underlying specificity of proteins targeting inorganic materials. Nano letters 2006, 6 (3), 515–519. [DOI] [PubMed] [Google Scholar]
- 115.Hnilova M; Liu X; Yuca E; Jia C; Wilson B; Karatas AY; Gresswell C; Ohuchi F; Kitamura K; Tamerler C, Multifunctional Protein-Enabled Patterning on Arrayed Ferroelectric Materials. ACS Applied Materials & Interfaces 2012, 4 (4), 1865–1871. [DOI] [PubMed] [Google Scholar]
- 116.Naik RR; Brott LL; Clarson SJ; Stone MO, Silica-precipitating peptides isolated from a combinatorial phage display peptide library. Journal of nanoscience and nanotechnology 2002, 2 (1), 95–100. [DOI] [PubMed] [Google Scholar]
- 117.Oliver D; Michaelis M; Heinz H; Volkov VV; Perry CC, From phage display to structure: an interplay of enthalpy and entropy in the binding of the LDHSLHS polypeptide to silica. Physical chemistry chemical physics : PCCP 2019, 21 (8), 4663–4672. [DOI] [PubMed] [Google Scholar]
- 118.Sanghvi AB; Miller KP-H; Belcher AM; Schmidt CE, Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nature materials 2005, 4 (6), 496–502. [DOI] [PubMed] [Google Scholar]
- 119.Sinensky AK; Belcher AM, Biomolecular Recognition of Crystal Defects: A Diffuse‐Selection Approach. Advanced Materials 2006, 18 (8), 991–996. [Google Scholar]
- 120.Tamerler C; Dincer S; Heidel D; Zareie MH; Sarikaya M, Biomimetic multifunctional molecular coatings using engineered proteins. Progress in Organic Coatings 2003, 47 (3–4), 267–274. [Google Scholar]
- 121.Tamerler C; Khatayevich D; Gungormus M; Kacar T; Oren EE; Hnilova M; Sarikaya M, Molecular biomimetics: GEPI-based biological routes to technology. Biopolymers 2010, 94 (1), 78–94. [DOI] [PubMed] [Google Scholar]
- 122.Vreuls C; Zocchi G; Genin A; Archambeau C; Martial J; Van de Weerdt C, Inorganic-binding peptides as tools for surface quality control. Journal of inorganic biochemistry 2010, 104 (10), 1013–1021. [DOI] [PubMed] [Google Scholar]
- 123.Cetinel S; Caliskan HB; Yucesoy DT; Donatan AS; Yuca E; Urgen M; Karaguler NG; Tamerler C, Addressable self-immobilization of lactate dehydrogenase across multiple length scales. Biotechnol J 2013, 8 (2), 262–72. [DOI] [PubMed] [Google Scholar]
- 124.Hnilova M; Karaca BT; Park J; Jia C; Wilson BR; Sarikaya M; Tamerler C, Fabrication of hierarchical hybrid structures using bio-enabled layer-by-layer self-assembly. Biotechnol Bioeng 2012, 109 (5), 1120–30. [DOI] [PubMed] [Google Scholar]
- 125.VanOosten SK; Yuca E; Karaca BT; Boone K; Snead ML; Spencer P; Tamerler C, Biosilver nanoparticle interface offers improved cell viability. Surf Innov 2016, 4 (3), 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wisdom C; VanOosten SK; Boone KW; Khvostenko D; Arnold PM; Snead ML; Tamerler C, Controlling the Biomimetic Implant Interface: Modulating Antimicrobial Activity by Spacer Design. J Mol Eng Mater 2016, 4 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Y; Snead ML; Tamerler C, Bio-inspired hard-to-soft interface for implant integration to bone. Nanomedicine 2015, 11 (2), 431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang S; Karaca BT; VanOosten SK; Yuca E; Mahalingam S; Edirisinghe M; Tamerler C, Coupling Infusion and Gyration for the Nanoscale Assembly of Functional Polymer Nanofibers Integrated with Genetically Engineered Proteins. Macromol Rapid Commun 2015, 36 (14), 1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yucesoy DT; Hnilova M; Boone K; Arnold PM; Snead ML; Tamerler C, Chimeric peptides as implant functionalization agents for titanium alloy implants with antimicrobial properties. JOM (1989) 2015, 67 (4), 754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuca E; Karatas AY; Seker UO; Gungormus M; Dinler-Doganay G; Sarikaya M; Tamerler C, In vitro labeling of hydroxyapatite minerals by an engineered protein. Biotechnol Bioeng 2011, 108 (5), 1021–30. [DOI] [PubMed] [Google Scholar]
- 131.Ye Q; Spencer P; Yuca E; Tamerler C, Engineered Peptide Repairs Defective Adhesive-Dentin Interface. Macromol Mater Eng 2017, 302 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yazici H; O’Neill MB; Kacar T; Wilson BR; Oren EE; Sarikaya M; Tamerler C, Engineered Chimeric Peptides as Antimicrobial Surface Coating Agents toward Infection-Free Implants. ACS Appl Mater Interfaces 2016, 8 (8), 5070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yazici H; Habib G; Boone K; Urgen M; Utku FS; Tamerler C, Self-assembling antimicrobial peptides on nanotubular titanium surfaces coated with calcium phosphate for local therapy. Mater Sci Eng C Mater Biol Appl 2019, 94, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yazici H; Fong H; Wilson B; Oren EE; Amos FA; Zhang H; Evans JS; Snead ML; Sarikaya M; Tamerler C, Biological response on a titanium implant-grade surface functionalized with modular peptides. Acta Biomater 2013, 9 (2), 5341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wisdom C; Chen C; Yuca E; Zhou Y; Tamerler C; Snead ML, Repeatedly Applied Peptide Film Kills Bacteria on Dental Implants. JOM (1989) 2019, 71 (4), 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gungormus M; Fong H; Kim IW; Evans JS; Tamerler C; Sarikaya M, Regulation of in vitro calcium phosphate mineralization by combinatorially selected hydroxyapatite-binding peptides. Biomacromolecules 2008, 9 (3), 966–73. [DOI] [PubMed] [Google Scholar]
- 137.Yuca E; Karatas AY; Seker UOS; Gungormus M; Dinler-Doganay G; Sarikaya M; Tamerler C, In Vitro Labeling of Hydroxyapatite Minerals by an Engineered Protein. Biotechnology and Bioengineering 2011, 108 (5), 1021–1030. [DOI] [PubMed] [Google Scholar]
- 138.Ye Q; Spencer P; Yuca E; Tamerler C, Engineered Peptide Repairs Defective Adhesive–Dentin Interface. Macromolecular Materials and Engineering 2017, 302 (5), 1600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh V; Misra A; Parthasarathy R; Ye Q; Spencer P, Viscoelastic properties of collagen–adhesive composites under water‐saturated and dry conditions. Journal of Biomedical Materials Research Part A 2015, 103 (2), 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Teo AJT; Mishra A; Park I; Kim YJ; Park WT; Yoon YJ, Polymeric Biomaterials for Medical Implants and Devices. Acs Biomater Sci Eng 2016, 2 (4), 454–472. [DOI] [PubMed] [Google Scholar]
- 141.Khan F; Tanaka M; Ahmad SR, Fabrication of polymeric biomaterials: a strategy for tissue engineering and medical devices. J Mater Chem B 2015, 3 (42), 8224–8249. [DOI] [PubMed] [Google Scholar]
- 142.Studenovska H; Slouf M; Rypacek F, Poly(HEMA) hydrogels with controlled pore architecture for tissue regeneration applications. J Mater Sci-Mater M 2008, 19 (2), 615–621. [DOI] [PubMed] [Google Scholar]
- 143.Zhao X; Wu YB; Du YZ; Chen XF; Lei B; Xue YM; Ma PX, A highly bioactive and biodegradable poly(glycerol sebacate)-silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration. J Mater Chem B 2015, 3 (16), 3222–3233. [DOI] [PubMed] [Google Scholar]
- 144.Becker ML; Liu JQ; Wooley KL, Functionalized micellar assemblies prepared via block copolymers synthesized by living free radical polymerization upon peptide-loaded resins. Biomacromolecules 2005, 6 (1), 220–228. [DOI] [PubMed] [Google Scholar]
- 145.Glinel K; Jonas AM; Jouenne T; Leprince J; Galas L; Huck WTS, Antibacterial and Antifouling Polymer Brushes Incorporating Antimicrobial Peptide. Bioconjugate Chem 2009, 20 (1), 71–77. [DOI] [PubMed] [Google Scholar]
- 146.Kim K; Lee M; Park H; Kim JH; Kim S; Chung H; Choi K; Kim IS; Seong BL; Kwon IC, Cell-permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J Am Chem Soc 2006, 128 (11), 3490–3491. [DOI] [PubMed] [Google Scholar]
- 147.Dalsin JL; Hu BH; Lee BP; Messersmith PB, Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc 2003, 125 (14), 4253–4258. [DOI] [PubMed] [Google Scholar]