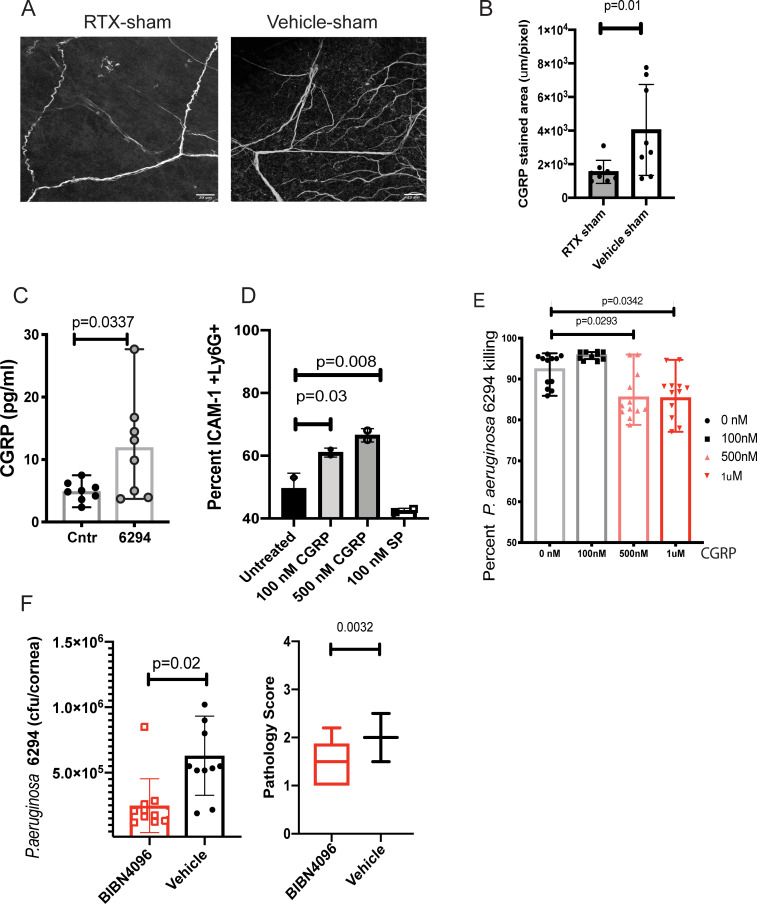
Fig 7. CGRP inhibits bactericidal activities of neutrophils.
A. RTX treatment reduces CGRP levels in sham-treated corneas. Representative images of CGRP staining of corneal whole-mounts from RTX and vehicle-treated shams. Z-stacks were collected from cornea periphery using Zeiss LSM710 confocal microscope with a 40x objective, scale bar = 20 μm. The z-stacks were collapsed to 2D and images were analyzed using Fiji software to quantify stained areas per image. The Z-stacks comprised sub-basal nerve plexus and stromal nerves spanning a depth of 40μm. Eight whole mounts per group per treatment were analyzed (Student’s t-test, p = 0.01). Each value represents an individual animal. B. The quantification of CGRP stained areas shows a decrease of CGRP staining in the sub-basal neuronal fibers of cornea periphery of RTX sham-treated mice when compared to sham vehicles. C. P. aeruginosa 6294 infections significantly induced CGRP release in trigeminal ganglia (TG)-derived neuronal cultures. CGRP concentrations were measured in the supernatants by ELISA. Bars represent means of individual mice values (symbols; Student’s t-test, p = 0.03). Saline treatment was used as control. Data are representative of two experiments. D. Exposure to CGRP, but not Substance P, upregulates membrane ICAM-1 in BM-derived neutrophils exposed to P. aeruginosa. PMNs were pretreated with 100 nM, 500 nM CGRP, or 100nM Substance P for 6h, then exposed to P. aeruginosa 6294 at MOI 0.01 for 30 min. Cells were washed, Fc blocked, and stained for ICAM-1+, CD11b+, Ly6G+, and 7AAD. Viable, Ly6G positive cells were compared for ICAM-1 levels. Percent ICAM-1+ cells were plotted. Data represent mean values. The experiment was repeated twice. E. CGRP inhibits bactericidal function of neutrophils. Data from three independent experiments are combined; each symbol represents cells derived from an individual mouse. One-way ANOVA, Dunnett’s multiple comparison test, p = 0.02 and p = 0.03. F. CGRP antagonist decreases bacterial burden in the infected corneas and conjunctival tissues. C57BL/6 mice were infected with P. aeruginosa 6294 at 5x105 CFU/eye. BIBN4096 (30 mg/kg) or vehicle were injected intraperitoneally one hour after the infectious challenge. Corneal and conjunctival tissues were harvested at 24h. Symbols represent individual mice. 10 mice per cohort were analyzed. Vehicle-treated mice had significantly higher bacterial presence when compared to BIBN4096-treated mice, illustrating CGRP-driven inhibition of immunity to P. aeruginosa (Student’s t-test, p = 0.02) (first plot). BIBN4096 treatment moderately reduced corneal pathology in the infected mice (Mann-Whitney, p = 0.003). Cumulatively, data show that P. aeruginosa induces CGRP release by neuronal cells in vitro and that blockade of CGRP in vivo partially resembles the phenotype of the infected RTX-treated mice displaying lower bacterial presence during early hours of infection. The phenotype is likely due to reduced opsonophagocytic killing in the presence of CGRP and correlates with upregulation of ICAM-1 in neutrophils.