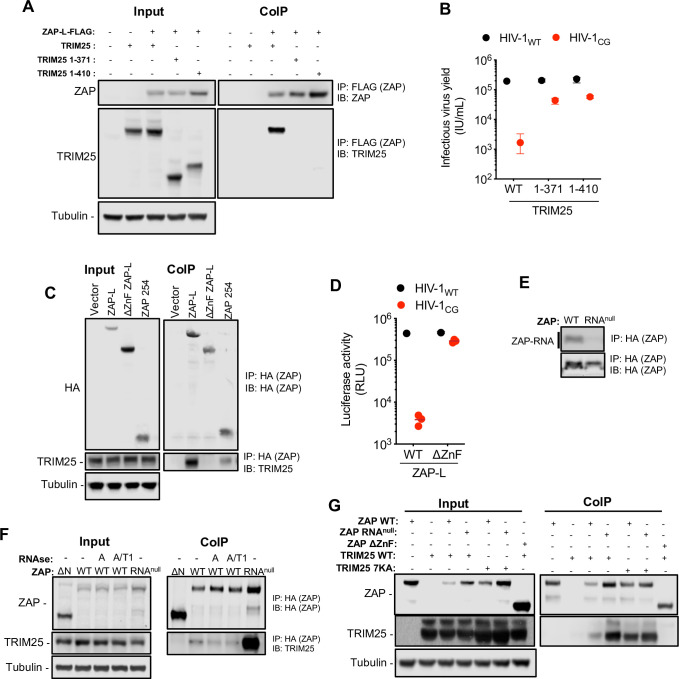
Fig 5. TRIM25 binds the ZAP NTD independently of RNA.
(A) HEK293T ZAP-/- and TRIM25 -/- cells were co-transfected with plasmids expressing human ZAP-L-FLAG and full-length untagged human TRIM25 or one of two human TRIM25 truncations (corresponding to the N-terminal 371 or 410 amino acids of TRIM25), that lack the SPRY domain [21]. Proteins were immunoprecipitated from cell lysates with an anti-FLAG antibody and subjected to western blot analysis. IP, immunoprecipitation. IB, immunoblot. (B) HEK293T ZAP-/- TRIM25 -/- cells were co-transfected with proviral plasmids and plasmids encoding human ZAP and full-length TRIM25 or the indicated truncated TRIM25 proteins. Infectious virus yield was determined using MT4-R5-GFP target cells. (C) HEK293T ZAP-/- cells were transfected with an empty vector or plasmids encoding human ZAP-L-HA, a truncated ZAP lacking the RNA binding domain (ΔZnF ZAP-L) or a truncated ZAP comprising the N-terminal 254 amino acids (ZAP 254). Proteins were immunoprecipitated from cell lysates with an anti-HA antibody and analysed by western blotting to detect overexpressed ZAP-L-HA and endogenous TRIM25. (D) Antiviral activity of ΔZnF ZAP-L was assessed by co-transfecting HEK293T ZAP-/- cells with indicated proviral plasmids and plasmids encoding full length (WT) or truncated (ΔZnF) human ZAP. Infectious virus yield was determined using MT4-R5-GFP target cells. (E) HEK293T ZAP-/- cells were transfected with plasmids encoding human ZAP-L (WT) or an RNA-binding mutant of ZAP (RNAnull, R74A, R75A, K76A). After overnight incubation cells were irradiated with UV light, and ZAP proteins were immunoprecipitated. RNA bound to each protein was radiolabelled and protein-RNA adducts were resolved by SDS-PAGE, transferred to a nitrocellulose membrane and exposed to autoradiographic film. In parallel, a western blot of immunoprecipitated proteins was done using anti-HA antibody. IP, immunoprecipitation. IB, immunoblot. (F) HEK293T ZAP-/- cells were transfected with plasmids encoding HA-tagged human ZAP-L (WT), ΔZnF ZAP-L or ZAP (RNAnull). Cell lysates were treated with RNAse A or a mixture of RNAse A and T1. ZAP protein complexes immunoprecipitated and subjected to western blot analysis to detect overexpressed ZAP-L-HA and endogenous TRIM25. (G) HEK293T ZAP-/- and TRIM25 -/- cells were transfected with plasmids encoding HA-tagged ZAP-L (WT), ΔZnF ZAP-L or ZAP (RNAnull), along with untagged TRIM25 or a TRIM25 RNA binding mutant (TRIM25 7KA). ZAP-HA protein complexes immunoprecipitated and subjected to western blot analysis to detect overexpressed ZAP and TRIM25 proteins.