Figure 2.
Inflammatory mechanisms, alveolar epithelial and endothelial damage, and coagulopathy in COVID-19
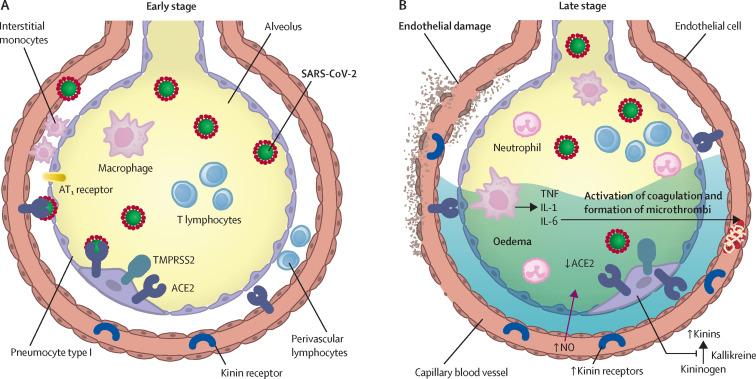
(A) In the early stage of disease, SARS-CoV-2 infects the bronchial epithelial cells as well as type I and type II alveolar pneumocytes and capillary endothelial cells. The serine protease TMPRSS2 promotes viral uptake by cleaving ACE2 and activating the SARS-CoV-2 S-protein. During early infection, viral copy numbers can be high in the lower respiratory tract. Inflammatory signalling molecules are released by infected cells and alveolar macrophages, in addition to recruited T lymphocytes, monocytes, and neutrophils. (B) With disease progression, plasma and tissue kallikreins release vasoactive peptides known as kinins that activate kinin receptors on the lung endothelium, which in turn leads to vascular smooth muscle relaxation and increased vascular permeability. This process is controlled by the ACE2 receptor. Without ACE2 blocking the ligands of kinin receptor B1, the lungs are prone to vascular leakage, angioedema, and downstream activation of coagulation. Dysregulated proinflammatory cytokine (TNF, IL-1, IL-6) and NO release and signalling contribute to these processes. As a consequence, pulmonary oedema fills the alveolar spaces, followed by hyaline membrane formation, compatible with early-phase acute respiratory distress syndrome. Anomalous coagulation frequently results in the formation of microthrombi and subsequent thrombotic sequelae. ACE2=angiotensin-converting enzyme 2. AT1 receptor=type 1 angiotensin II receptor. IL=interleukin. NO=nitric oxide. TMPRSS2=transmembrane protease serine 2. TNF=tumour necrosis factor.