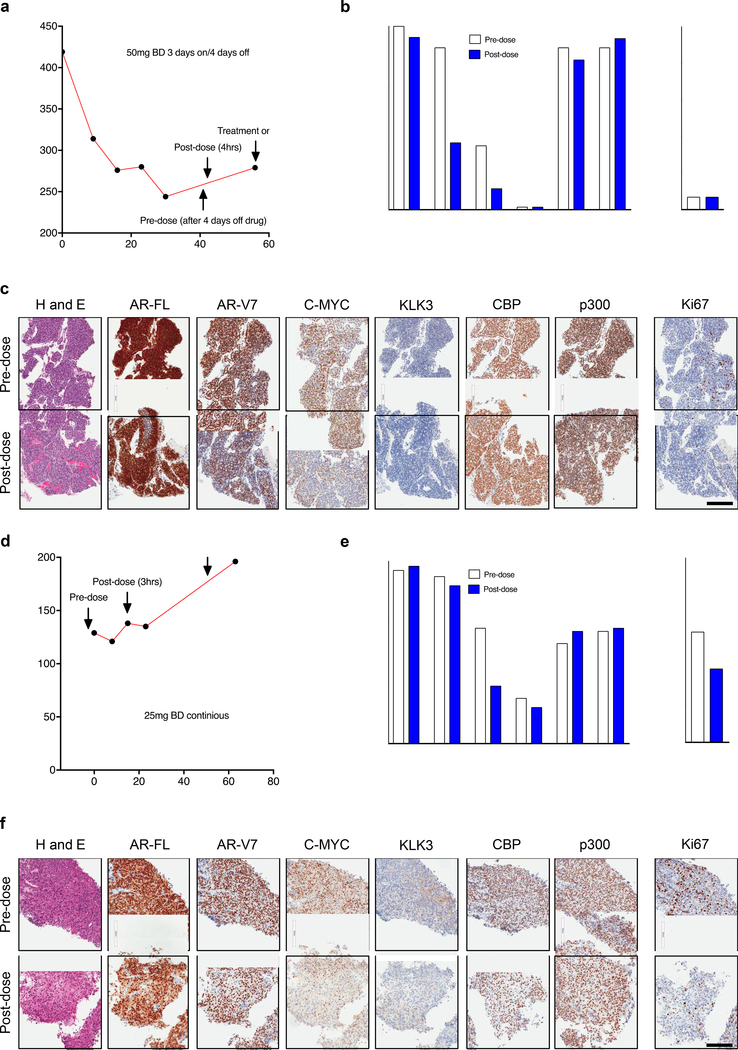
Figure 7: CCS1477 regulates key prostate cancer therapeutic targets and modulates blood KLK3 in patients with castration resistant prostate cancer.
(a-f) Patient 1 (a-c) and patient 2 (d-f) were treated with CCS1477 within an ongoing Phase I clinical trial (NCT03568656). Patient 1 (a-c) was treated orally with 50mg twice daily (BD) on a 3- days-on-and-4 days-off-schedule. Blood KLK3 levels and timing of pre-dose (after 4-days off treatment) and post-dose (4-hours post-CCS1477 dose) biopsies are shown (a). The patient remains on trial. Quantification (b) and representative micrographs for haematoxylin and eosin (H and E), AR-FL, AR-V7, C-MYC, KLK3, CBP, p300 and Ki67 immunohistochemistry pre-dose and post-dose are shown (c). Scale bar represents 200 μm. Patient 2 (d-f) was treated orally with 25mg BD continuously. Blood KLK3 levels and timing of pre-dose (prior to starting treatment) and post-dose (3 hours post CCS1477 dose) biopsies are shown (d). The patient has stopped treatment. Quantification (e) and representative micrographs for H and E, AR-FL, AR-V7, C-MYC, KLK3, CBP, p300 and Ki67 immunohistochemistry pre-dose and post-dose are shown (f). Scale bar represents 200 μm.