Abstract
Cell death occurs when a pathogen invades a host organism or the organism is subjected to sterile injury. Thus, cell death is often closely associated with the induction of an immune response. Furthermore, cell death can occur as a consequence of the immune response and precedes the tissue renewal and repair responses that are initiated by innate immune cells during resolution of an immune response. Beyond immunity, cell death is required for development, morphogenesis and homeostasis. How can such a ubiquitous event as cell death trigger such a wide range of context-specific effector responses? Dying cells are sensed by innate immune cells using specialized receptors and phagocytosed through a process termed efferocytosis. Here, we outline a general principle whereby signals within the dead cell as well as the environment are integrated by specific efferocytes to define the appropriate effector response.
Keywords: Biological sciences, Immunology, Cell death and immune response, [URI /631/250/1933], Inflammation, [URI /631/250/256]
Table of contents
Cell death recognition can result in a multitude of distinct effector responses. Here, the authors discuss a framework for determining the specific effector response to cell death that relies on its recognition, contextual environmental signals and identity of the efferocyte.
Introduction
The main function of the immune system is self versus non-self discrimination and defence against foreign invaders while avoiding, as much as possible, self-inflicted damage to host tissues. In these processes, immune cells can both kill and die. On the one hand, CD8+ T cells and natural killer (NK) cells actively kill infected cells or altered host cells, and macrophages can induce cell suicide or apoptosis (BOX 1)1,2. On the other hand, immune cells such as activated T cells die during the contraction phase of the immune response3,4. Furthermore, immune cells such as macrophages and dendritic cells (DCs) are involved in the recognition and removal of dead cells resulting from injury and infection5, from homeostatic processes such as the routine death of senescent red blood cells6, or from embryonic development7,8. Thus, cell death is closely associated with the functions of the immune system.
Box 1 | Overview of the major types of cell death and their molecular mechanisms.
Here, we outline the major programmed cell death modalities discussed in this Review. For a more detailed description, we refer the reader to REF.46.
Apoptosis
Stimuli inducing intrinsic apoptosis results in the formation of mitochondrial permeability transition pores and the release of cytochrome c into the cytoplasm. Cytochrome c binds to APAF1 inducing its oligomerization, thereby forming the apoptosome. The apoptosome activates the initiator caspase, caspase 9, which in turn activates the executioner caspases, caspase 3 and caspase 7. Cellular targets such as laminin, PARP and DNA are cleaved. Additionally, the release of SMAC/DIABLO from mitochondria neutralizes the suppressive effects of inhibitor of apoptosis proteins (IAPs), promoting the activation of executioner caspases. In extrinsic apoptosis, activation of death receptors of the tumour necrosis factor (TNF) family, including TNF receptor 1 (TNFR1), FAS (also known as CD95) and TNF-related apoptosis-inducing ligand (TRAIL) receptors 1 and 2 (also known as TNFRSF10A and TNFRSF10B), leads to formation of the death-inducing signalling complex (DISC), which activates the initiator caspase, caspase 8. Caspases cleave ATP11C, an enzyme that flips phosphatidylserine (PtdSer) from the outer leaflet to the inner leaflet of the plasma membrane and activate XKR8, an enzyme that moves PtdSer to the outer leaflet of the plasma membrane. This allows for exposure of PtdSer on the outer leaflet of the plasma membrane of apoptotic cells. PtdSer functions as an ‘eat me’ signal recognized by various receptors on neighbouring cells, thus promoting the engulfment of the apoptotic cell by the efferocyte.
Necroptosis
The activation of receptor-interacting serine/threonine-protein kinase 3 (RIPK3) by RIPK1, downstream of FAS, TNFR1, Toll-like receptor or interferon receptor signaling, drives necroptosis. The activation of RIPK3 by RIPK1 occurs when caspase 8 activation is suppressed and results in the formation of the necrosome complex [G]. RIPK3-dependent phosphorylation of mixed lineage kinase domain-like protein (MLKL) results in MLKL pore assembly at the plasma membrane. MLKL pores promote the flux of calcium, sodium and potassium ions. Necroptosis is generally associated with sterile inflammation. Several damage-associated molecular patterns (DAMPs), such as high-mobility group box 1 (HMGB1) and ATP, are released during necroptosis. Additionally, inflammasome activation leads to release of proinflammatory cytokines and chemokines. Necroptotic cells are known to expose PtdSer and to be engulfed by macrophages as well as fibroblasts. It is unknown whether specific pathways exist for the recognition and clearance of necroptotic cells.
NETosis
In NETosis, the dying neutrophil releases a neutrophil extracellular trap (NET) composed of decondensed chromatin studded with anti-microbial proteins such as neutrophil elastase and myeloperoxidase (MPO) that traps and kills pathogens. Neutrophil elastase, MPO and NADPH oxidase are required for the production of reactive oxygen species (ROS) and/or DNA decondensation during NET formation. NETosis can be stimulated by complement components and complement opsonization. In turn, NETs may activate complement as well as the clotting cascade and platelet aggregation. Removal of NETs requires macrophages.
Pyroptosis
Pyroptosis is observed primarily in macrophages and their precursors. Activation of caspase 1 and caspase 11 in mice results in the cleavage and activation of gasdermin D (GSDMD). The amino-terminal GSDMD fragment, GSDMD-N, oligomerizes to form pores in the plasma membrane. Various caspases cleave and enable the secretion of IL-1 family cytokines (such as IL-1β and IL-18) and also cause the release of DAMPs such as lactate dehydrogenase and HMGB1. Pyroptotic cells are known to expose PtdSer. Macrophages and fibroblasts have been described to engulf pyroptotic cells.
Ferroptosis
Ferroptosis is dependent on free iron or iron-containing lipoxygenase enzyme [Au:OK?] and is defined by the accumulation of ROS, the oxidation of polyunsaturated fatty acid-containing phospholipids and the loss of lipid peroxide repair capacity driven by phospholipid hydroperoxidase GPX4. PtdSer exposure has been described in ferroptosis and macrophages were shown to be capable of engulfing ferroptotic cells.
Necrosis
Necrosis is an accidental, passive cell death, such as when killed by heat or during osmotic lysis. The morphological hallmark of necrotic cells is rupture of the cell membrane, swelling of organelles and poring out of cytoplasmic components. Among the released substances are DAMPs that induce an inflammatory response. Macrophages have been shown to engulf necrotic cells.
It is clear that the failure of cells to die has serious deleterious immune consequences for organisms. Mice defective in the cell death signalling pathway mediated by FAS (also known as CD95) develop lymphadenopathy, splenomegaly and autoimmune disease9–11. In humans, mutations in the genes encoding FAS, caspase 8 or caspase 10 are associated with autoimmune lymphoproliferative syndrome12–15. The genetic ablation of BCL-2-interacting mediator (BIM), a pro-apoptotic protein, results in excessive numbers of lymphoid and myeloid cells and autoimmune disease16. What is less clear is the relationship between cell death, its recognition and disposal and the specificity of the subsequent effector responses. For example, cells such as neutrophils die by apoptosis during tissue repair responses after injury17. Normally, apoptotic cell death is associated with tissue repair and not inflammation18. However, apoptotic cell death during sterile tissue damage or infection can induce inflammation, either alone or accompanied by the activation of the adaptive immune response19,20. Thus, apoptosis can be associated with both non-inflammatory and inflammatory effector responses. The homeostatic cell death of red blood cells can trigger their clearance by splenic red pulp macrophages, but this is associated with neither tissue repair nor inflammation, albeit the neighbouring white pulp of the spleen is an epicentre for adaptive immunity and the marginal zone is involved in both innate and adaptive immunity21,22.
The array of effector responses to cell death is large, including many forms of immune defence, distinct tissue maintenance functions and developmental functions such as digit formation and neuronal circuit development. How can the complex and multidimensional information that results in such an array of effector responses be encoded in cell death? Can we unravel this complex code to predict the effector responses to cell death that shape our biology? The concept that different types of cell death result in specific effector responses is not new but here, we discuss the possibility that the cell type that engulfs the dead cell during its removal, known as the efferocyte, integrates signals encoded by both the dead cell and the environment of cell death to execute a specific effector response (FIG. 1). The efferocyte is typically a professional phagocyte of the immune system such as a macrophage or DC, although in some cases, the efferocyte may be a non-professional phagocyte such as a fibroblast or endothelial cell. Notwithstanding, we propose that the efferocyte holds the secret to the specificity of the effector response when a cell dies.
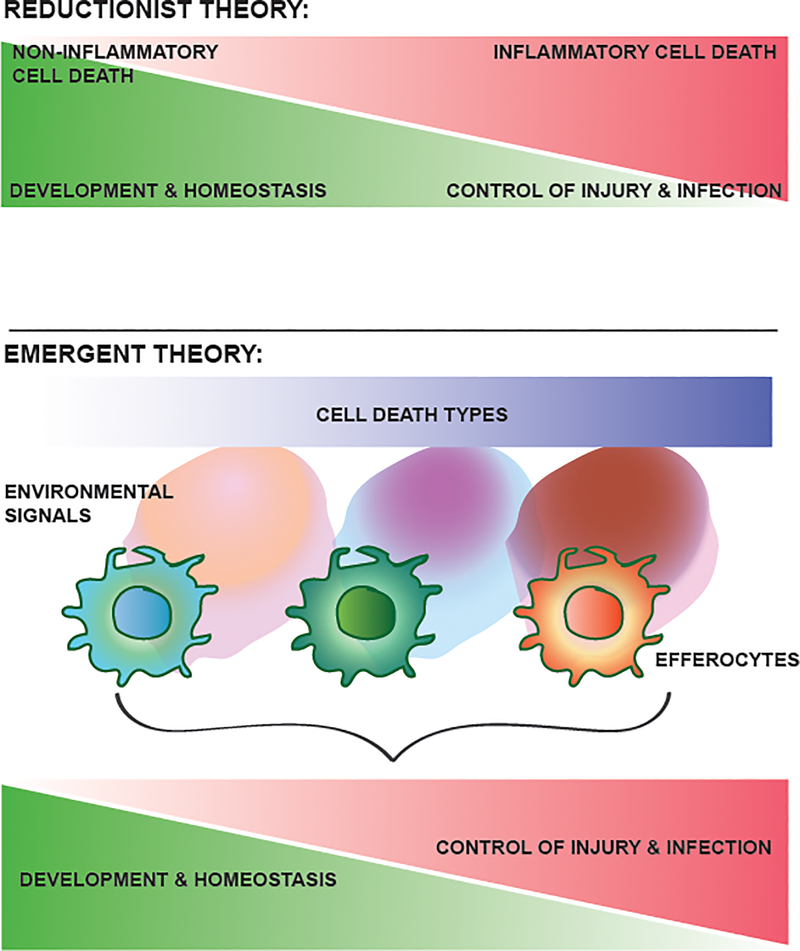
Figure 1 |. Schematic representation of the proposed code of cell death.
According to a reductionist theory (top), there is a one-to-one relationship between the type of cell death and the effector function. As an example, non-inflammatory cell death is associated with physiological resolution and repair responses, whereas inflammatory cell death is associated with the control of injury and infection but can result in pathologies associated with defective resolution or repair. By contrast, the emergent theory (bottom) that we outline in this Review takes into account the variety of cell death types, the multitude of accompanying environmental signals and their integration by different efferocytes for the execution of specific effector responses. As such, the same cell death modality can result in distinct effector functions, depending on the specificity of environmental signals and the efferocyte. For simplicity, only three types of cell death, environment, efferocyte and effector response are shown here, but in the broader contexts of development, homeostasis and repair, there are a large set of spatially and temporally distinct combinations resulting in different effector responses.
The reductionist approach
In the simplest scenario, there could be a one-to-one correlation of cell death with effector function, such that the biological consequences of cell death are carried out by the act of death itself. In theory, a specific form of programmed cell death driven by a distinct molecular mechanism could encode a unique effector function. For example, cell death during development or homeostasis is primarily apoptotic23–27 and is not associated with antimicrobial activity or inflammation. By contrast, death by NETosis (BOX 1) of neutrophils during an infection ensures the ensnarement of invading bacteria and other pathogens within DNA nets28. Cell death during Salmonella infection can occur by pyroptosis (BOX 1) and is associated with significant inflammation29. A corollary, then, would be that the degree of inflammation associated with a specific form of programmed cell death correlates with physiological versus pathological outcomes of cell death (FIG. 1).
However, the number of types of cell death is fewer than the number of unique effector responses such that a single type of cell death is not restricted to a single effector function, which argues against a one-to-one correlation between cell death and effector function. Apoptosis has been categorized as generally anti-inflammatory and pyroptosis has been deemed inflammatory. However, death by apoptosis or pyroptosis can elicit multiple effector responses. Apoptotic death during development30,31 and during homeostatic cellular turnover in adults32,33 is not associated with inflammation, whereas apoptotic death during infection or in sterile tissue damage does induce inflammation19,20. Apoptotic death in the context of infection can activate the adaptive immune response, whereas in the context of sterile injury it could be limited to inflammation19,20. It should also be noted that components of the molecular machinery of apoptosis, such as caspase 3, have recently been implicated in inflammatory cell death through the activation of gasdermin E34. Thus, apoptosis is associated with both non-inflammatory and inflammatory forms of cell death and with several, distinct effector responses35. A similar relationship has recently been described for pyroptosis. This form of cell death is mostly associated with infection and the production of pro-inflammatory mediators such as IL-1 and IL-18, but a recent study reported the requirement of pyroptosis in neurodevelopment36. Pyroptosis triggered in a sterile environment through the sensing of neuronal DNA damage resulted in the elimination of neurons in a gasdermin-D-dependent manner36. Of note, IL-1 or IL-18 signalling were not required for this developmental function of pyroptosis36. Therefore, a reductionist approach of dissecting cell death to its molecular mechanisms does not seem to hold all of the clues to the effector response.
Integrating cell death and clearance
To understand the consequences of cell death for an organism, here we posit considering cell death together with the cell type responsible for its disposal. Cell death is closely associated with its recognition and clearance, and the effector function associated with cell death is manifested during its disposal. For example, resolution of pulmonary inflammation is associated with the engulfment and clearance of apoptotic neutrophils by macrophages17. Similarly, apoptotic cells introduced intravenously while grafting bone marrow in mice are removed by host macrophages, which facilitates engraftment of the donor bone marrow37.
The engulfment of dead cells by phagocytes (known as efferocytosis) is frequently observed in homeostasis32. Phosphatidylserine (PtdSer) is displayed on the outer membrane leaflet of apoptotic cells and is recognized by receptors that initiate efferocytosis38,39. Sequestering PtdSer by the microinjection of a PtdSer-binding protein in seminiferous tubules of mice resulted in the accumulation of apoptotic cells and decreased number of spermatogenic cells and epididymal sperms40. The failure of efferocytosis during development can be associated with morphogenetic defects. In Drosophila, RNA interference of croquemort (which encodes a hemocyte and macrophage receptor for apoptotic cells) resulted in central nervous system defects41. The inhibition of macrophage function during amphibian or mammalian development also leads to morphogenetic and developmental defects42,43. A clear example of the integration of cell death with its clearance is seen in Caenorhabditis elegans. The combination of a mutation that renders cells partially defective in undergoing cell death (ced-3 partial loss of function) with mutations in engulfment genes (ced-1, ced-2, ced-5, ced-6, ced-7, ced-10 and ced-12) leads to the survival of cells that are otherwise destined to die and the presence of supernumerary cells in the organism44,45.
Thus, in contrast to a reductionist view that cell death encodes information specifying the effector programme to be carried out by a passive responder, we propose that cell death requires the participation of an active intermediary to execute the encoded tasks and that the effector response can be understood in terms of a composite of the nature of cell death, the cellular responders to cell death and the context of cell death (FIG. 1). In this regard, we posit three factors that determine the effector response, which can be described by the formulae given in BOX 2. First, is the identity of the cell that recognizes and engulfs the dying cell. Second, is the information content of the dying cell beyond the molecular mechanisms responsible for cell death, which may involve shared molecules that are displayed or released by dying cells as well as unique signals present in the specific dying cell. Third, is the contextual framework of cell death, which involves the integration of signals from surrounding living tissue as well as from the dead cell. Every factor represents a set of variables, but a sequence of constraints at each level limits the outcomes. For example, approximately fifteen distinct molecular mechanisms of programmed cell death have been described46, some of which are exclusively triggered in specific environments (for example, pyroptosis producing IL-1 and IL-18 in the setting of infection and inflammation)47. Thus, the environment can constrain the information encoded in cell death. Constraints can also occur at the level of the efferocyte that engulfs the dead cell. The metabolic and/or transcriptional capabilities of distinct efferocytes, such as infiltrating monocyte-derived macrophages, tissue-resident macrophages or epithelial cells, restrict their tissue distribution and effector responses. Finally, we posit that the whole may be different than the sum of its parts. The nature of the efferocyte not only constrains the possible responses but also is the operational system within which integration can occur. Thus, the effector response is not merely selected as one of several possible outcomes specified by the dying cell or by the environment alone but also can be different from these outcomes. In summary, collective information provided by the type of cell death, environment and effector cell, together with a multifactorial series of constraints, is integrated to specify a single output (BOX 2). Below, we develop this integrated theory to provide a rough guide for the study of the physiological and pathological effector responses to cell death.
Box 2 | A mathematical model for effector responses to cell death.
Classical understanding of the effector response
Cell death can occur by multiple molecular mechanisms. Information regarding how the cell died is contained within the dead cell. In addition, there may be information regarding whether the cell is infected or uninfected, and even about its specific identity. Let be the set of ‘types of programmed cell death’, let be the set of ‘identities of the dying cell’ and let be the set of ‘other variables’. Then, the set of ‘cell deaths’ can be described formally as the Cartesian product shown in equation (1.1).
| (1.1) |
In other words, a type of ‘programmed cell death’, an ‘identity of the dying cell’ and ‘other variable’ completely characterize a type of ‘cell death’. The simplest response to cell death occurs where death itself executes the programmed function and, hence, the ‘effector response’ is a direct function of ‘cell death’. Formally, if we denote as the set of ‘effector responses’, then a type of ‘cell death’ can give rise to exactly one type of ‘effector response’ and thus be described as the function given in equation (1.2).
| (1.2) |
That is, a type of ‘cell death’ can be thought to be assigned to a unique type of ‘effector response’ .
Proposed theory of the effector response
Our proposition is that the classical understanding of the ‘effector response’ as a function of ‘cell death’ given by equation (1.2) is incomplete because a type of ‘cell death’ can be observed to give rise to multiple ‘effector responses’. Instead, we take into account the environment of cell death and the specific efferocyte involved in disposing of the dead cell, and we propose a new understanding of ‘effector responses’ as a function of these additional inputs. More precisely, if we denote as the set of ‘environments of cell death’ and as the set of ‘specific efferocytes’ then we propose the following new understanding of the ‘effector response’ as a function of ‘cell death’, ‘environment of cell death’ and ‘specific efferocyte’ given by equation (2.1).
| (2.1) |
In other words, a type of ‘cell death’, a type of ‘environments of cell death’ and a type of ‘specific efferocyte’ give rise to a unique type of ‘effector response’.
In some special cases, the environment of cell death and the type of cell death can be constrained, for example pyroptosis and inflammation are obligatorily paired. The environment may also pre-specify the type of efferocyte that is available to recognize and dispose of the dead cell. These constraints can be considered by a simple extension of equation (2.1). Namely, there is a subset , which we refer to as the set of ‘admissible combinations’, for which the “effector response” can be understood as a function of the set of ‘admissible combinations’, as given by equation (2.2).
| (2.2) |
In other words, in equation (2.2), a type of “cell death”, a type of ‘environments of cell death’ and a type of ‘specific efferocyte’ give rise to a unique type of ‘effector response’ if the triple is an ‘admissible combination’.
The role of the efferocyte
The efferocyte has a crucial role in determining the effector response to cell death (FIG. 2). Living neighbouring cells carry out specific effector functions that are necessary for the development or survival of the tissue and/or organism following a cell death event. In C. elegans, dead cells are removed by their sessile neighbours48. However, in most phyla, disposal is mainly carried out by professional phagocytes known as macrophages. DCs are also capable of efferocytosis of apoptotic cells and infected apoptotic cells19. That said, several other cell types, including epithelial cells, fibroblasts and endothelial cells, are also known to engulf dead cells49–52. In fact, it has been proposed that most cells can function as efferocytes in some circumstances53. Here, we postulate that the identity of the efferocyte, at least partially, determines a composite response to cell death.
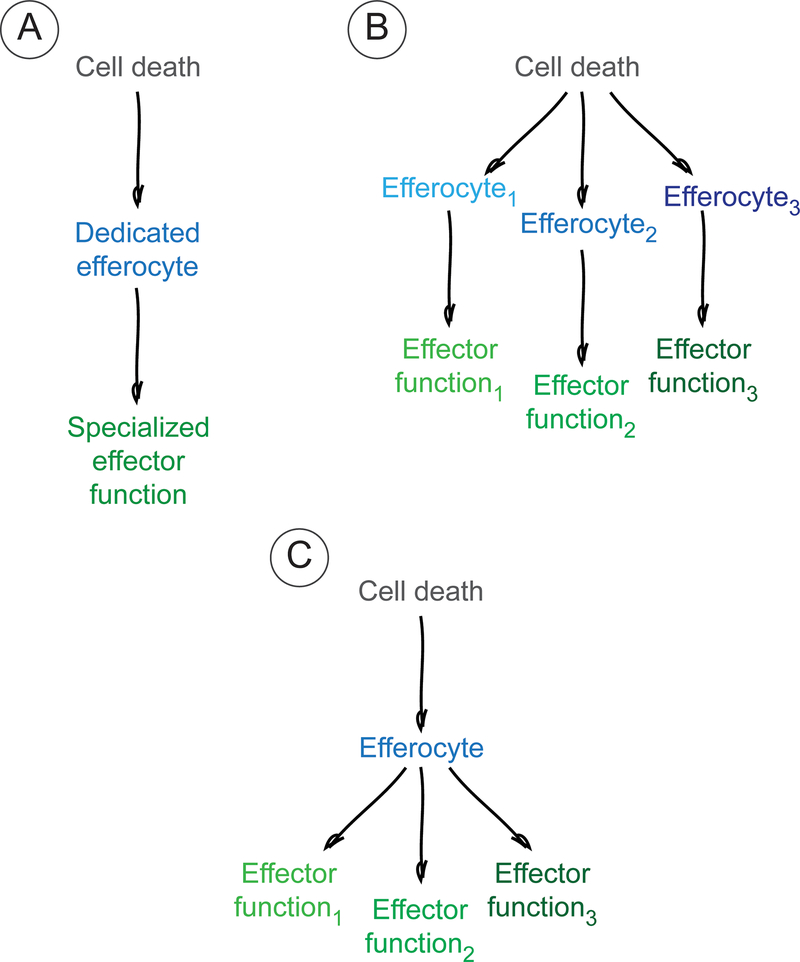
Figure 2 |. The role of the efferocyte.
Three distinct scenarios represent possible relationships between cell death, efferocytes and effector responses. a | Recognition of a particular type of dead cell by a dedicated efferocyte results in a specialized effector function. b,c | Alternatively, the recognition of dead cells could give rise to multiple and distinct effector functions. This could result from the recognition of dead cells by different efferocytes (b) or from divergent effector responses being executed by the same efferocyte (c).
One efferocyte, one response.
It is possible that a primary type of efferocyte is designated for a specific cell type that is programmed to die (FIG. 2a). The prevalence of a designated primary efferocyte at various points in development or at different locations in tissues can respond to the cell death that is destined to occur in time and space. In turn, the identity of the efferocyte may define the effector response. In the brain, microglia are the professional efferocytes, but neuronal progenitors, glial precursors and astrocytes can also efferocytose neuronal and axonal debris54–60. Microglia are present in the developing brain from embryonic day 9 (E9)–E10 in mice61. One study estimated that 70% and 50% of cortical cells, primarily progenitor cells, die at E14 and E18, respectively, during mouse brain development62. Thus, the microglia are poised to remove cells that die during early brain development. By contrast, cortical astrocytes begin to emerge only around E16–E18, with the bulk of them emerging around postnatal day 7 (P7)61. Co-incidentally, a second, larger wave of programmed cell death of specific post-mitotic neuronal populations is observed in the cortex in the first to second week after birth63. Therefore, astrocytes as well as microglia are available to clear these dying post-mitotic cells. The assignment of a specialized efferocyte to a specific population of dying cells during development may pair a specific effector function to the death of those cells. In the case of neural development, the specific effector function is unknown. However, one example of a dedicated pairing of type of efferocyte with dying cell type, leading to a specialized effector function, is that of splenic red pulp macrophages, which engulf dying red blood cells and metabolize the iron that they contain64.
Multiple efferocytes, different responses.
Alternatively, different efferocytes may be involved in the disposal of a particular dying cell type, resulting in distinct effector responses (FIG. 2b). For example, both alveolar macrophages, which are professional phagocytes, and bronchial epithelial cells engulf apoptotic cells in lung airways49. Although the consequences of efferocytosis by these cell types remain to be directly described, it was reported that alveolar macrophages release insulin-like growth factor 1 (IGF1) while phagocytosing apoptotic cells, which in turn reduces efferocytosis by bronchial epithelial cells50. Thus, efferocytosis by non-professional phagocytes can be tuned by professional phagocytes. Presumably, this redirects efferocytosis to macrophages and perhaps instructs the physiologically appropriate response. Although bronchial epithelial cells, similarly to macrophages, can suppress inflammation in an IL-10-dependent manner following efferocytosis49, the absence of IGF1 receptor on bronchial epithelial cells resulted in exacerbated lung inflammation after allergen exposure50. Another example of multiple efferocytes clearing a particular dead cell type occurs in the context of the intestine. Apoptotic intestinal epithelial cells are engulfed by a subset of DCs and two distinct subsets of macrophages, each of which has a unique gene expression signature that suggests a specialized effector function65. The two macrophage subsets upregulate genes involved in phagosome maturation, lipid metabolism and branched-chain amino acid catabolism after efferocytosis65. By contrast, DCs upregulate genes involved in regulatory T (Treg) cell recruitment and proliferation, and CC-chemokine receptor 7 (CCR7)65. Similarly, both fibroblasts (human dermal, lung or kidney) and macrophages can engulf apoptotic neutrophils in vitro51,66, although the resulting effector functions are unknown. Furthermore, the effector response following phagocytosis by differentially polarized macrophages can be expected to be dissimilar if one considers their polarization states as being at opposite ends of a gene expression spectrum.
One efferocyte, different responses.
Although different cell types can partake in efferocytosis and thereby contribute to the diversity in effector responses, even the same efferocyte can mediate different responses (FIG. 2c). For example, macrophages can support the restoration of homeostasis and regeneration, as well as scarring. During hepatocyte regeneration following chronic liver injury, the efferocytosis of hepatocyte debris by macrophages and the resultant release of WNT3A promote specification of hepatic progenitor cells into hepatocytes67. By contrast, macrophage depletion during carbon tetrachloride-induced liver fibrosis results in reduced scar formation68. Similarly, following punch biopsy injuries in the skin of mice, early depletion of macrophages (in the inflammatory phase) results in delayed wound healing but decreases the formation of scar tissue69.
How can the same efferocyte respond differently? The diversity of responses from identical efferocytes may result from the large number of efferocytosis receptors and downstream pathways. In C. elegans, two parallel pathways of efferocytosis have been described70. In mammals, the best known efferocytosis receptors are PtdSer sensors. PtdSer is recognized directly by several receptors including BAI1 (also known as ADGRB1), TIMD4, CD300LF, STAB1 and STAB238. Furthermore, exposed PtdSer can be tethered indirectly to TAM (TYRO3, AXL and MERTK) receptor tyrosine kinases or to MEGF10 through the soluble ligands GAS6 and PROS1 or C1q, respectively38.
The interpretation that different receptors may endow an efferocyte with functional specialization is consistent with tissue-resident macrophage populations having heterogeneous expression of phagocytic receptors71. Perhaps more surprising is that the same cell type can express multiple efferocytosis receptors at the same time. For example at P21 in mice, liver-resident macrophages express Timd4 and Mertk, whereas kidney-resident macrophages express high levels of Timd4 and Stab172. Human dermal fibroblasts use vitronectin receptor or a mannose/fucose-specific lectin for efferocytosis51. Although tissue-resident macrophages and fibroblasts are unlikely to be a homogenous species, analyses of single-cell RNA-sequencing data indicate that a single efferocyte can indeed express multiple efferocytosis receptors. For example, AXL and TIMD4 are both expressed by individual mouse liver macrophages (see the VirtualCytometry website).
Assuming that the expression of multiple receptors by a single cell is not simply built-in redundancy, this observation suggests that the engagement of a specific receptor or pathway may signal a distinct effector response. For example, two different efferocytosis pathways have been described for the removal of Corazonin-expressing neurons in the ventral nerve cord (vCrz+ cells) of Drosophila larvae. Whereas Draper was required for the elimination of vCrz+ cell bodies, the Crk–Mbc–dCed-12 pathway, but not Draper, was required for the removal of vCrz+ neurites. Interestingly, draper mutants, but not mutants of the Crk–Mbc–dCed-12 axis, had preserved and morphologically intact vCrz+ neurons73. These results suggest that Draper-dependent phagocytosis may promote neuronal apoptosis73, whereas the Crk–Mbc–dCed-12 pathway may be involved in circuit remodelling. Another example is provided by MERTK, which is expressed by retinal pigmented epithelial cells in the eye and by Sertoli and Leydig cells in the testes74,75. Mertk–/– mice are characterized by retinal degeneration, and male Mertk–/–Axl–/– mice or Mertk–/–Tyro3–/– mice have reduced testis weight, sperm count and fertility75,76. The expression of BAI1 in Mertk–/– mice rescues the clearance of apoptotic germ cells in the testes but fails to prevent or reduce photoreceptor degeneration77, which points to redundancies, as well as functional specializations, between these two efferocytosis receptors. Both BAI1 and MERTK can participate in the efferocytosis of apoptotic germ cells, but only MERTK and not BAI1 can function in the efferocytosis of photoreceptors. Although in this example two different cell types are involved, theoretically the phagocytic removal of a dead cell by two distinct receptors within a single cell could be coupled to the production of discrete sets of downstream factors.
In summary, there are at least three ways in which cell death can be paired with particular efferocytes to specify the appropriate effector response. There could be a dedicated primary efferocyte assigned to respond to programmed cell death occurring at a precise time and place, which would allow for the evolution of specialized effector functions. A downside of this system could be the lack of an alternative means of disposal such that functional deficits in the efferocyte are likely to be fatal or associated with severe disease. Alternatively, different efferocytes might be available to dispose of the dead cell, but they are organized hierarchically or must crosstalk to establish a dynamic hierarchy. Each efferocyte has a fixed effector function, thus the hierarchy determines the effector function. Finally, a single efferocyte could be capable of distinct effector responses, perhaps resulting from the choice of receptor it uses to sense the dead cell. These latter two scenarios allow for specialization with a degree of failsafe. Although the failsafe measures may not be complete substitutes of the required function, some degree of redundancy or compensation may be possible, which would reduce the mortality or severity of the diseases associated with defects in a particular type of efferocytosis.
The role of the dying cell
The second factor in our theory is the information contained in the dying cell (FIG. 3). For an in-depth review of the different types of cell death, which we do not intend to cover here, the reader is referred to REF.46. If the identity of the efferocyte is some kind of an operational environment for decoding information, the source code is represented by the dying or dead cell.
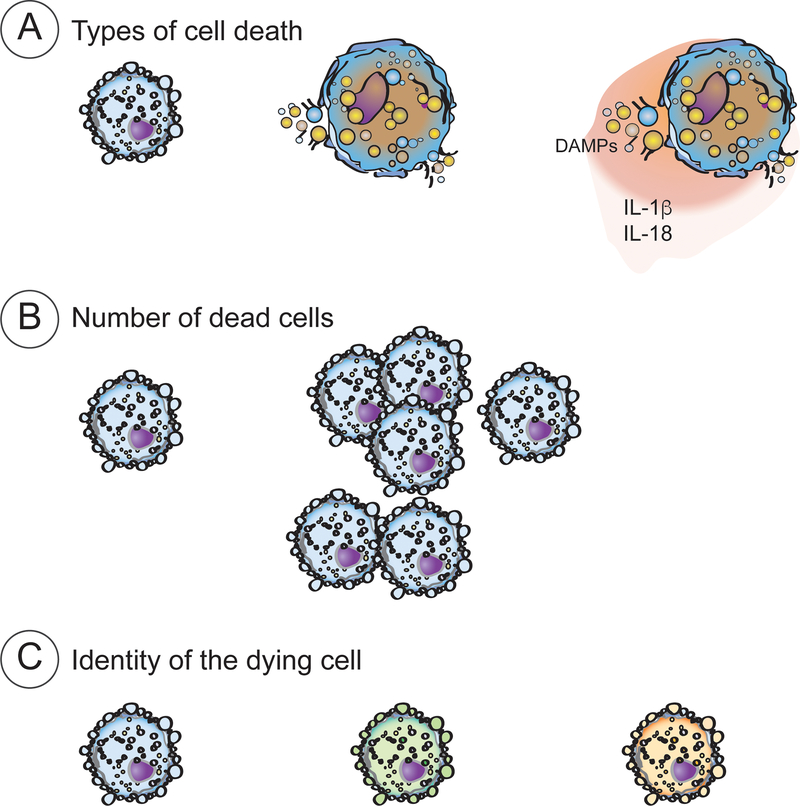
Figure 3 |. The role of the dying cell.
The information provided by the dying cell can be contained in the type of cell death modality, the number of dead cells and the identity of the dead cells.
Type of cell death.
It has been previously described that the molecular machineries of specific forms of cell death can specify the appropriate effector response, such as in the case of immunogenic versus tolerogenic responses78,79. The specific molecular modality of cell death by NETosis can trap and kill bacteria28. Pyroptotic death of macrophages can generate structures known as pore-induced intracellular traps that trap and damage live bacteria80. Moreover, these dead macrophages together with the trapped bacteria provide specific signals for complement and scavenger receptor activation, and the recruitment of macrophages and neutrophils for effective bacterial killing80. A specific functional relationship between pyroptosis and anti-bacterial effector responses can also be inferred from the elaborate defence mechanisms in bacteria that are dedicated to avoiding inflammasome activation and hence pyroptosis81,82. IL-18, which is released during pyroptosis, can also induce IFNγ and T helper 1 (TH1) cell responses83, and the other pyroptosis-associated cytokine IL-1β can promote TH17 cell differentiation84,85. Admittedly, IL-1β and IL-18 are not synonymous with inflammasome activation and inflammasome activation can occur without pyroptosis86, but this form of cell death may contain some information to generate a specific anti-bacterial effector response. However, it is important to note that pyroptosis is not exclusive to bacterial infections and can also occur following viral infections, Alzheimer disease or liver disease and even during neuronal development36,87–89.
Another form of programmed cell death, necroptosis (BOX 1), is associated with the release of danger signals in the form of damage-associated molecular patterns (DAMPs) such as ATP and high-mobility group box 1 (HMGB1). It is likely that cellular sensors of PtdSer functioning together with cytokine receptors or DAMP sensors translate pyroptotic or necroptotic cell death into a specific effector response or lack of response. HMGB1 has been reported to inhibit efferocytosis by competing with PtdSer for receptor binding, as well as by sequestering αvβ3 integrins on the surface of phagocytes90,91. Finally, lactic acidosis associated with acute tumour lysis syndrome, which results from lactate production following the loss of mitochondrial membrane potential during apoptosis92, has been shown to directly affect macrophage polarization and effector function93.
Therefore, specific forms of cell death can contain information for specifying the effector response. Interestingly, infection can change the mode of cell death and, thus, the information coded therein. Intracellular community-associated methicillin-resistant Staphylococcus aureus suppresses the apoptosis of neutrophils and inhibits their efferocytosis by macrophages. This redirects infected neutrophils to undergo programmed necrosis94 (BOX 1). Whether this change in the type of cell death directly affects the effector response is not known, but macrophage effector responses after S. aureus infection are reprogrammed to produce more IL-8 (also known as CXCL8) and less tumour necrosis factor (TNF) and IL-1β94.
Plasticity between cell death modalities.
The programmed cell death of a single cell does not occur through a predetermined route and plasticity between different modalities of cell death has recently been uncovered95,96. For example, TNF signalling can induce apoptosis or necroptosis through caspase 8 or receptor-interacting serine/threonine-protein kinase 3 (RIPK3), respectively97. Interestingly, although Ripk3−/− and Mlkl−/− mice, which have defective necroptosis, develop normally98–100, Casp8 deletion, which prevents apoptosis, results in embryonic lethality owing to excessive necroptosis101–103. Thus, one form of cell death (apoptosis) keeps at bay the other (necroptosis) and its associated deleterious consequences. Implicit in this phenomenon is the idea that the modality of cell death codes for specificity of the effector response. Whether or how the efferocytes involved interpret embryonic apoptosis versus necroptosis to execute the appropriate effector response remains unknown.
Number of dead cells.
The number of cells that die at a time could also contain quantitative information that is coordinated by efferocytes to mediate the appropriate response. For example, macrophages can organize a predictive response to the extent of injury; plucking a certain density of hair from mouse skin can stimulate the regrowth of plucked as well as unplucked hairs, but plucking hairs below this threshold density does not result in hair regeneration104. Even if the same number of hairs were plucked, the area from which they were plucked was crucial. It is not known whether the role of macrophages in sensing dead cells plays a role in this process, but it is likely that a combination of cytokines, chemokines and macrophages are involved104. Macrophages can phagocytose cargoes sequentially. Apoptotic cells can be engulfed by macrophages through LC3-associated phagocytosis [G] (LAP). It was noted that apoptotic cell engulfment leads to increased mitochondrial fission in macrophages, which was necessary for increased cytosolic levels of calcium, following LAP-associated calcium release from endoplasmic reticulum. In turn, cytosolic calcium was necessary for recycling of the phagolysomal membrane after the first round of phagocytosis and for complete sealing of the phagosome for engulfment of the cargo during the second LAP event. This enabled repeated uptake of apoptotic cells by the same macrophage105. Disabling mitochondrial fission inhibited repeated efferocytosis105.
Identity of dead cells.
The identity of the dead cell may also determine the specific effector function. Although macrophages are generally thought to be agnostic to the identity of dying cells, macrophage engulfment specifically of dead type II alveolar epithelial cells in the lung has been identified as a trigger for lung fibrosis106,107. Similarly, dead cells can contain information regarding whether they died from an infection or otherwise. DC-mediated efferocytosis of uninfected apoptotic cells led to Treg cell differentiation, whereas DC-mediated efferocytosis of infected apoptotic cells in the gut of mice infected with Citrobacter rodentium led to TH17 cell differentiation19. These results indicate that DCs not only recognize uninfected and infected apoptotic cells as distinct identities, but also respond in a manner that is specific and appropriate. These observations suggest that the information embedded in a dead cell likely extends beyond hallmarks such as PtdSer exposure and involves unique features, at least for some cells. These unique features, including pathogen-associated molecular patterns (PAMPs) or DAMPs, may engage different receptors in the efferocyte and underlie the distinct effector responses. Indeed, IL-1 family DAMPs (including IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ) can differentially direct TH cell polarization to shape the adaptive immune response108, and IL-18 or IL-33 can induce Treg cells to produce amphiregulin and trigger lung tissue repair after influenza virus infection109. These cytokines function in sterile inflammation and also synergize with PAMPs to amplify infection-associated inflammation108.
The role of the environment
The final factor to determine the response to cell death is the contextual frameworks in which cell death and its sensing occur. We posit that the tissue environment can directly regulate the effector response of the efferocyte either through specialized cytokines that are uniquely present in an environment, or through unique tissue-specific epigenetic and/or transcriptional changes or unique metabolic functions associated with a specific environment (FIG. 4). Integrating multiple coincidental signals in an environment-specific context could potentially limit the possible effector responses of macrophages from a broader generic repertoire to a specific subset of tasks. Finally, large-scale changes in effector functions and efferocytosis are observed in aging tissue.
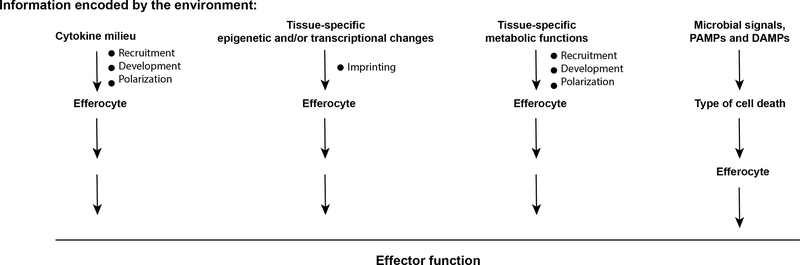
Figure 4 |. The role of the environment.
The tissue environment can contribute to determining the effector response of the efferocyte either directly through specialized cytokines that are uniquely present in an environment, or through unique tissue-specific epigenetic and/or transcriptional changes or unique metabolic functions associated with a specific environment, or indirectly by influencing the type of cell death, which in turn can affect the effector response.
Specialized cytokine environment.
The tissue environment can shape the response to dead cells by specifying the identity of the efferocyte, which in turn determines the effector response. For example, keratinocytes produce IL-34, which is required for the differentiation of Langerhans cells110. Langerhans cells are a locally self-renewing subset of DCs found in skin111, together with other efferocytes such as dermal DCs, classical DCs and macrophages. Despite the presence of a large number of different efferocytes in skin, Langerhans cells specifically are necessary for the generation of antigen-specific-TH17 cell responses112. By contrast, Langerin+ dermal DCs are required for the generation of antigen-specific cytotoxic T lymphocytes112.
Mammary stem cells were shown to require the support of macrophages recruited by colony-stimulating factor 1 (CSF1)113. Homozygous loss of function of CSF1 resulted in failure to recruit macrophages to developing mammary glands and a severe reduction in the ability of mammary stem cells to repopulate mammary glands114. Thus, CSF1-mediated recruitment of macrophages shapes mammary gland morphogenesis. Whether this effector function involves macrophage-mediated efferocytosis remains unknown; however, involution or apoptotic death of mammary epithelial cells and their removal by non-professional epithelial cells and professional macrophages are known to precede repopulation of the mammary gland by differentiation of mammary stem cells into adipocytes115,116.
A more direct role of cytokines together with cell death sensing in shaping the expression of tissue-repair genes is shown by the example that macrophages trigger tissue repair upon exposure to IL-4 together with apoptotic cells, but not IL-4 or apoptotic cells alone18. There are other examples of the effect of the tissue environment on macrophage functions, although in these cases the role of cell death was not explored. For example, surfactant protein A in the lungs following helminth-induced lung tissue damage, and C1q in the liver following Listeria monocytogenes infection, function as local amplifiers of IL-4-dependent macrophage-mediated tissue repair117. Comparing a biological material (urinary bladder matrix) with a synthetic material (polycaprolactone), it was shown that whereas urinary bladder matrix promotes tissue repair through a TH2 cell- and IL-4-mediated programme, polycaprolactone drives CD9+IL-36γ+ macrophages towards a TH17 cell- and IL-17-mediated programme, resulting in fibrosis118.
Tissue-specific enhancers and transcription factors.
Tissue environments are well known to shape the distinct phenotypes of resident or recruited macrophage subsets by differentially regulating enhancer elements and the expression of transcription factors119. For example, monocytes recruited to the liver following Kupffer cell ablation differentiate into Kupffer cells under the combined influence of transforming growth factor-β and bone morphogenetic protein 2 produced by liver sinusoidal endothelial cells120. Similarly, imprinting of naive macrophages with the alveolar macrophage fate requires their spatial proximity with lung basophils121. Large cavity macrophages that populate peritoneal, pleural and pericardial spaces require a WT1+ mesothelial and fibroblastic niche for their maintenance122. Thus, the dedicated effector functions of these specialized efferocytes are shaped by their tissue environment.
Intriguingly, at least in some cases, efferocytes remain capable of differential gene expression when removed from the specified environment. Lung alveolar macrophages were reported to be intrinsically less responsive to IL-4 compared with lung interstitial macrophages or peritoneal cavity macrophages123, but lung alveolar macrophages can regain IL-4 responsiveness once removed from the lung environment123. The insensitivity to IL-4 was driven by the distinct metabolic state of lung alveolar macrophages in the lung environment123.
Functional changes in the effector responses of aging macrophages may result from the epigenetic and/or metabolic alterations that occur in these cells. Commonly, pro-inflammatory cytokine production as well as MHC class II and CD86 expression are increased with aging124,125. Aged macrophages are also less effective as efferocytes125. Aging tissues are characterized by several generic features, including genomic instability, epigenetic changes, protein misfolding coupled with a reduced unfolded protein response, and autophagy and mitochondrial dysfunction124,126. Other mechanisms might also be involved. Reduced efferocytosis by aged macrophages correlated with a reduction in MERTK expression, through cleavage of this receptor127. This was associated with a skewing towards the production of leukotriene B4 by macrophages instead of resolvin D1127.
Commensals and other extrinsic signals.
The sensing of cell death by efferocytes is often integrated with external cues such as PAMPs and DAMPs. As described earlier, apoptosis in the absence of microbial signals induces Treg cell differentiation, whereas apoptosis associated with C. rodentium infection resulted in TH17 cell differentiation19. In addition, it was shown that phagocytosis of Escherichia coli-infected neutrophils by DCs lacking Myd88 and Trif or Tlr4, which have defects in PAMP sensing, led to Treg cell differentiation instead of TH17 cell differentiation19. Another paradigm described earlier regarding plasticity between apoptosis and necroptosis during embryonic development can be further extended to include pyroptosis. Whereas Casp8−/−Mlkl−/− mice, which have defects in apoptosis and necroptosis, are viable103, Casp8C326A/C326AMlkl−/− mice, which have a catalytically inactive caspase 8 mutation, die during perinatal development128, at least in part owing to pyroptosis and inflammation-associated damage in the intestine95,96. Importantly, this phenomenon is restricted mostly to the gut, as endothelial cells or skin epithelial cells were not susceptible to pyroptosis. Thus, plasticity of cell death and the associated response might be determined through the integration of an environmental microbial signal. It will be interesting to learn if the pyroptotic death seen in Casp8C326A/C326AMlkl−/− intestine is also observed for mice bred in gnotobiotic facilities.
Conclusions and future directions
It remains a mystery how a unique effector response from amongst the multitude of possible effector responses to cell death is selected in response to a specific event of cell death to ensure that the physiological requirements of the tissue and/or organism are fulfilled. Here, we propose an integrated approach, primarily focusing on the efferocyte as a compiler of the source code that is written in the death of a cell. Thus, cell death undoubtedly contains invaluable information, but additionally requires an intermediate for the execution of the programme encoded by this information. We also include the environmental context as an additional factor that can influence the effector response by determining the form of cell death or by affecting the efferocyte. Together, these components define a cell death code that directs the effector functions associated with cell death (BOX 2).
What could be the importance of proposing this code of cell death? We believe that this theoretical construct can reveal unique specificities of the immune response to different inducers as well as explaining the dysregulation of the magnitude and resolution of the immune response when the code goes awry. For example, necroptosis has been proposed to evolve as a developmental checkpoint that aborts fetuses with severe developmental abnormalities97. This form of cell death also promotes adaptive immunity, for example during pneumococcal or viral infections129,130. Yet, necroptosis is associated with several diseases that might have in common an aetiology of dysregulated inflammation97,131,132. The improper execution and/or recognition of necroptosis may contribute to dysregulation of the magnitude of the immune response in relapsing–remitting diseases such as inflammatory bowel disease or even in neurodegenerative diseases.
Another potential outcome of a cell death code may be a principle that allows us to understand the difference in the type of immune response that is associated with, for example, a viral infection compared with sterile tissue damage — in other words, the quality of the immune response. It is possible that a combination of the type of cell death and its recognition by efferocytes underlies at least some of the differences in the effector response in these settings. At least functionally, antigen presentation is a crucial discriminating factor between infection and sterile injury and therefore it is likely that the recognition of cell death by professional antigen-presenting cells such as DCs is more relevant in the context of an infectious injury, whereas macrophages may be more strongly associated with sterile injuries.
In cancer, malignant cells can die by apoptosis, necroptosis or ferroptosis (BOX 1). Furthermore, apoptotic cancer cells can be phagocytosed by DCs, monocyte-derived infiltrating macrophages, tissue-resident macrophages or even fibroblasts133. The consequences of efferocytosis of tumour corpses by DCs or macrophage subsets are not completely characterized. One study identified CD103+ DCs, but not CD11b+ DCs, as crucial effector cells for the uptake and presentation of tumour antigens for T cell priming in the lymph nodes and generation of tumour-infiltrating T cells134. Perhaps the redirection of efferocytosis towards CD103+ DCs could be used to boost the anti-tumour response. Fibroblasts normally lack mechanisms for the presentation of antigens, although antigen presentation is upregulated under certain conditions such as in response to IFNγ signalling135,136. Thus, fibroblast-mediated phagocytosis of tumour corpses may lead to either immune evasion or the presentation of tumour-derived antigens, depending on the cytokine environment. Therefore, it is possible that the identity of the efferocyte and the environmental signals contribute to determining whether or not an anti-tumour immune response will be induced.
Changes to the cell death code — be the type of cell death, the efferocyte that encounters the dead cell or the cytokine environment, or an integration of several events — might also regulate the period of the immune response by encoding the timing of the transition from inflammation to resolution, which is a crucial step in the recovery of tissue function following injury. Insufficient, as well as excessive, tissue repair damages mucosal tissue and impairs gastrointestinal function in inflammatory bowel disease and other intestinal diseases such as radiation enteritis and chronic ischemic enteritis, ranging from ulcers, abscesses and fistulas to fibrosis and strictures137. Fibrosis in other organs such as the lung or the liver also presents significant clinical problems138,139. Cystic fibrosis is associated with bronchiectasis and is also known to cause colonic wall thickening, fibrotic colonopathy and strictures137,140,141. Chronic foot ulcers are a frequent complication of patients with long-term diabetes and up to 14–24% of these patients require amputations142. Therapeutic interventions to improve wound healing are extremely limited. Interestingly, the removal of dead cells (known as debridement) is one of the few known ways of improving the healing of non-infected wounds143.
Discovering a fundamental, generalizable principle for the regulation of the magnitude, quality and period of the immune response will help us to better understand a vast array of diseases, including inflammation-associated degenerative diseases, diseases characterized by uncontrolled or error-prone immune responses and/or wound repair, as well as anti-cancer immunity. Recently, the plasticity between different modalities of cell death, such as upon inhibition of caspase 8 enzymatic activity or its proteolytic processing, has been discovered95,96. Redirecting phagocytosis between effector cells may alter the rate of uptake of dead cells. Both microglia and astrocytes are capable of efferocytosis but microglia are three-times more efficient than astrocytes in the phagocytosis of apoptotic lymphocytes in vitro144. Furthermore, the efferocytosis rate of microglia can be modulated by cytokines — IFNγ stimulates efferocytosis whereas IL-4 reduces efferocytosis — whereas astrocyte efferocytosis is agnostic to cytokine exposure144. Perhaps, one day we will be able to toggle modes of cell death, the responding efferocytes and environmental signals for improved immune function in hitherto difficult to treat diseases.
Supplementary Material
Acknowledgements
C.V.R. and S.G. acknowledge Malay K. Basu for analyses and discussions of single-cell RNA-sequencing data. T.D.H. acknowledges Madeleine Chalfant for insightful discussions. This work was supported by grants from the National Institutes of Health (NIH-NIAID R01 AI089824 and NIH-NCI R01 CA212376) and Kenneth Rainin Foundation. C.V.R is a Howard Hughes Medical Institute Faculty Scholar.
Glossary
- LC3-associated phagocytosis (LAP)
A form of phagocytosis during which the canonical autophagy protein LC3 is conjugated to the phagosome to form the LAPosome. LC3 conjugation is crucial for phagosome maturation and acidification, through fusion with lysosomes, and the degradation of cargo
- Necrosome complex
An amyloid signaling complex assembled upon interaction and activation (phosphorylation) of RIPK3 and RIPK1. The necrosome leads to the phosphorylation of MLKL and induction of necroptosis
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Immunology thanks [Referee#1 name], [Referee#2 name] and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Related links
VirtualCytometry: https://www.grnpedia.org/cytometry
References
- 1.Lowin B, Peitsch MC & Tschopp J Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol 198, 1–24, (1995). [DOI] [PubMed] [Google Scholar]
- 2.Gordon S The macrophage: past, present and future. Eur. J. Immunol 37 Suppl 1, S9–17, (2007). [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R & Gray D Immunological memory and protective immunity: understanding their relation. Science 272, 54–60, (1996). [DOI] [PubMed] [Google Scholar]
- 4.Swain SL et al. From naive to memory T cells. Immunol. Rev 150, 143–167, (1996). [DOI] [PubMed] [Google Scholar]
- 5.Martinez J Prix Fixe: Efferocytosis as a Four-Course Meal. Curr. Top. Microbiol. Immunol 403, 1–36, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratosin D et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80, 173–195, (1998). [DOI] [PubMed] [Google Scholar]
- 7.Voss AK & Strasser A The essentials of developmental apoptosis. F1000Res 9, doi: 10.12688/f1000research.21571.1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkinson-Woolley J, Hughes D, Gordon S & Martin P Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J. Cell Sci 107 ( Pt 5), 1159–1167 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Theofilopoulos AN & Dixon FJ Murine models of systemic lupus erythematosus. Adv. Immunol 37, 269–390, (1985). [DOI] [PubMed] [Google Scholar]
- 10.Andrews BS et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J. Exp. Med 148, 1198–1215, (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roths JB, Murphy ED & Eicher EM A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J. Exp. Med 159, 1–20, (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher GH et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81, 935–946, (1995). [DOI] [PubMed] [Google Scholar]
- 13.Chun HJ et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399, (2002). [DOI] [PubMed] [Google Scholar]
- 14.Wang J et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 98, 47–58, (1999). [DOI] [PubMed] [Google Scholar]
- 15.Rieux-Laucat F et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268, 1347–1349, (1995). [DOI] [PubMed] [Google Scholar]
- 16.Bouillet P et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738, (1999). [DOI] [PubMed] [Google Scholar]
- 17.Cox G, Crossley J & Xing Z Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am. J. Respir. Cell. Mol. Biol 12, 232–237, (1995). [DOI] [PubMed] [Google Scholar]
- 18.Bosurgi L et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076, (2017).This study is the first to show that the integration of IL4/IL-13 cytokine receptor signaling with sensing of apoptotic neutrophils is required for the induction of tissue repair responses in macrophages.
- 19.Torchinsky MB, Garaude J, Martin AP & Blander JM Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 458, 78–82, (2009).This paper shows that the recognition of infected apoptotic cells leads to the induction of TH17 responses, while the response to apoptotic cells sans microbial signals induces the differentiation of regulatory T cells.
- 20.Brereton CF & Blander JM The unexpected link between infection-induced apoptosis and a TH17 immune response. J. Leukoc. Biol 89, 565–576, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mebius RE & Kraal G Structure and function of the spleen. Nat. Rev. Immunol 5, 606–616, (2005). [DOI] [PubMed] [Google Scholar]
- 22.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK & van Bruggen R Of macrophages and red blood cells; a complex love story. Front. Physiol 5, 9, doi: 10.3389/fphys.2014.00009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi Y & Miura M Programmed cell death in neurodevelopment. Dev. Cell 32, 478–490, (2015). [DOI] [PubMed] [Google Scholar]
- 24.Kuan CY et al. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22, 667–676, (1999). [DOI] [PubMed] [Google Scholar]
- 25.De Zio D, Giunta L, Corvaro M, Ferraro E & Cecconi F Expanding roles of programmed cell death in mammalian neurodevelopment. Semin. Cell. Dev. Biol 16, 281–294, (2005). [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94, 739–750, (1998). [DOI] [PubMed] [Google Scholar]
- 27.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA & Gruss P Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94, 727–737, (1998). [DOI] [PubMed] [Google Scholar]
- 28.Brinkmann V et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535, (2004).This is the first study describing the formation of neutrophil extracellular traps, an effector function of netosis.
- 29.Fink SL & Cookson BT Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol 8, 1812–1825, (2006). [DOI] [PubMed] [Google Scholar]
- 30.Fadeel B Programmed cell clearance. Cell. Mol. Life. Sci 60, 2575–2585, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franc NC Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: molecular mechanisms and physiological consequences. Front. Biosci 7, d1298–1313 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Arandjelovic S & Ravichandran KS Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol 16, 907–917, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blander JM The many ways tissue phagocytes respond to dying cells. Immunol. Rev 277, 158–173, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers C et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun 10, 1689, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidovich P, Kearney CJ & Martin SJ Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol. Chem 395, 1163–1171, (2014). [DOI] [PubMed] [Google Scholar]
- 36.Lammert CR et al. AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature 580, 647–652, (2020).This study reports the requirement for AIM2 inflammasome and Gasdermin-D, but not IL-1 or IL-18, in the response to genotoxic stress in neurons during development.
- 37.Kleinclauss F et al. Intravenous apoptotic spleen cell infusion induces a TGF-β-dependent regulatory T-cell expansion. Cell Death & Differentiation 13, 41–52, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata S Apoptosis and Clearance of Apoptotic Cells. Annu Rev Immunol 36, 489–517, (2018). [DOI] [PubMed] [Google Scholar]
- 39.Martin SJ et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med 182, 1545–1556, (1995).This landmark study reports the early externalization of phosphatidylserine in a wide array of murine and human cells undergoing apoptosis.
- 40.Maeda Y, Shiratsuchi A, Namiki M & Nakanishi Y Inhibition of sperm production in mice by annexin V microinjected into seminiferous tubules: possible etiology of phagocytic clearance of apoptotic spermatogenic cells and male infertility. Cell Death & Differentiation 9, 742–749, (2002). [DOI] [PubMed] [Google Scholar]
- 41.Franc NC, Dimarcq JL, Lagueux M, Hoffmann J & Ezekowitz RA Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 4, 431–443, (1996). [DOI] [PubMed] [Google Scholar]
- 42.Lang RA & Bishop JM Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74, 453–462, (1993). [DOI] [PubMed] [Google Scholar]
- 43.Diez-Roux G & Lang RA Macrophages induce apoptosis in normal cells in vivo. Development 124, 3633–3638 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Hoeppner DJ, Hengartner MO & Schnabel R Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412, 202–206, (2001). [DOI] [PubMed] [Google Scholar]
- 45.Reddien PW, Cameron S & Horvitz HR Phagocytosis promotes programmed cell death in C. elegans. Nature 412, 198–202, (2001).In these studies, Hoeppner et al. and Reddien et al. report the discovery that cells normally destined to die, intriguingly survive in C. elegans carrying mutations in engulfment genes.
- 46.Galluzzi L et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergsbaken T, Fink SL & Cookson BT Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol 7, 99–109, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sulston JE, Schierenberg E, White JG & Thomson JN The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol 100, 64–119, (1983). [DOI] [PubMed] [Google Scholar]
- 49.Juncadella IJ et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493, 547–551, (2013).This manuscript identifies bronchial epithelial cells as phagocytes for neighbouring apoptotic epithelial cells and inducers of anti-inflammatory responses.
- 50.Han CZ et al. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 539, 570–574, (2016).In this study, Han et al., report on the ability of engulfing macrophages to regulate the phagocytic capacity and inflammatory response of epithelial cells.
- 51.Hall SE, Savill JS, Henson PM & Haslett C Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J. Immunol 153, 3218–3227 (1994). [PubMed] [Google Scholar]
- 52.Ramirez-Ortiz ZG et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat. Immunol 14, 917–926, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S & Birge RB Efferocytosis. Curr. Biol 26, R558–R559, (2016). [DOI] [PubMed] [Google Scholar]
- 54.Lu Z et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat. Cell Biol 13, 1076–1083, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu HH et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci 12, 1534–1541, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen JV et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. U S A 108, 1176–1181, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morizawa YM et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun 8, 28, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loov C, Hillered L, Ebendal T & Erlandsson A Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS One 7, e33090, doi: 10.1371/journal.pone.0033090 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iram T et al. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci 36, 5185–5192, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damisah EC et al. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv 6, eaba3239, doi: 10.1126/sciadv.aba3239 (2020).Making use of elegant photochemical induction of death in a single neuron in the mouse brain, Damisah et al., report the ability of astrocytes to engulf small dendritic apoptotic bodies whereas microglia is found to engulf the soma and apical dendrites.
- 61.Reemst K, Noctor SC, Lucassen PJ & Hol EM The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci 10, 566, doi: 10.3389/fnhum.2016.00566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blaschke AJ, Staley K & Chun J Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development 122, 1165–1174 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Wong FK & Marin O Developmental Cell Death in the Cerebral Cortex. Annu. Rev. Cell. Dev. Biol 35, 523–542, (2019). [DOI] [PubMed] [Google Scholar]
- 64.Morioka S, Maueroder C & Ravichandran KS Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity 50, 1149–1162, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cummings RJ et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539, 565–569, (2016).This is an interesting study that defines the transcriptional changes of distinct subsets of intestinal dendritic cells and macrophages as a consequence of the homeostatic removal of apoptotic epithelial cells.
- 66.Savill JS et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest 83, 865–875, (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boulter L et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med 18, 572–579, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duffield JS et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest 115, 56–65, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lucas T et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol 184, 3964–3977, (2010). [DOI] [PubMed] [Google Scholar]
- 70.Gumienny TL & Hengartner MO How the worm removes corpses: the nematode C. elegans as a model system to study engulfment. Cell Death Differ. 8, 564–568, (2001). [DOI] [PubMed] [Google Scholar]
- 71.A-Gonzalez N et al. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med 214, 1281–1296, (2017).This study identifies distinct types of receptors, opsonins and transcription factors in the phagocytosis of apoptotic cells by tissue resident macrophages, underscoring the heterogeneity of this process across multiple tissues.
- 72.Mass E et al. Specification of tissue-resident macrophages during organogenesis. Science 353, doi: 10.1126/science.aaf4238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tasdemir-Yilmaz OE & Freeman MR Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 28, 20–33, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D’Cruz PM et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet 9, 645–651, (2000). [DOI] [PubMed] [Google Scholar]
- 75.Chen Y et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction 138, 655–666, (2009). [DOI] [PubMed] [Google Scholar]
- 76.Duncan JL et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest. Ophthalmol. Vis. Sci 44, 826–838, (2003). [DOI] [PubMed] [Google Scholar]
- 77.Penberthy KK et al. Context-dependent compensation among phosphatidylserine-recognition receptors. Sci. Rep 7, 14623, doi: 10.1038/s41598-017-15191-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green DR, Ferguson T, Zitvogel L & Kroemer G Immunogenic and tolerogenic cell death. Nat. Rev. Immunol 9, 353–363, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferguson TA, Choi J & Green DR Armed response: how dying cells influence T-cell functions. Immunol. Rev 241, 77–88, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorgensen I, Zhang Y, Krantz BA & Miao EA Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med 213, 2113–2128, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jorgensen I & Miao EA Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev 265, 130–142, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Y et al. A Bacterial Effector Reveals the V-ATPase-ATG16L1 Axis that Initiates Xenophagy. Cell 178, 552–566 e520, (2019). [DOI] [PubMed] [Google Scholar]
- 83.Dinarello CA IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy. Clin. Immunol 103, 11–24, (1999). [DOI] [PubMed] [Google Scholar]
- 84.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM & Stockinger B TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189, (2006). [DOI] [PubMed] [Google Scholar]
- 85.Sutton CE et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341, (2009). [DOI] [PubMed] [Google Scholar]
- 86.Rathinam VA & Fitzgerald KA Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 165, 792–800, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee S, Hirohama M, Noguchi M, Nagata K & Kawaguchi A Influenza A Virus Infection Triggers Pyroptosis and Apoptosis of Respiratory Epithelial Cells through the Type I Interferon Signaling Pathway in a Mutually Exclusive Manner. Journal of Virology 92, e00396–00318, doi: 10.1128/jvi.00396-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan MS et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell. Death. Dis 5, e1382, doi: 10.1038/cddis.2014.348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szabo G & Petrasek J Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol 12, 387–400, (2015). [DOI] [PubMed] [Google Scholar]
- 90.Liu G et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol 181, 4240–4246, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friggeri A et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am. J. Physiol. Cell. Physiol 299, C1267–1276, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tiefenthaler M et al. Increased lactate production follows loss of mitochondrial membrane potential during apoptosis of human leukaemia cells. Br. J. Haematol 114, 574–580, (2001). [DOI] [PubMed] [Google Scholar]
- 93.Colegio OR et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenlee-Wacker MC et al. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J. Immunol 192, 4709–4717, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Newton K et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682, (2019). [DOI] [PubMed] [Google Scholar]
- 96.Fritsch M et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687, (2019).In these two studies, Newton et al. and Fritsch et al., reveal the plasticity between and hierarchy across different cell death modalities when specific executionary mediators of cell death are inhibitied.
- 97.Shan B, Pan H, Najafov A & Yuan J Necroptosis in development and diseases. Genes Dev. 32, 327–340, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Newton K, Sun X & Dixit VM Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol 24, 1464–1469, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu J et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polykratis A et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol 193, 1539–1543, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaiser WJ et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oberst A et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alvarez-Diaz S et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity 45, 513–526, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen CC et al. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y et al. Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell 171, 331–345 e322, (2017).This study identified a metabolic pathway required for sequencial phagocytosis
- 106.Sisson TH et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med 181, 254–263, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim KK et al. Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis. Cell. Death Dis 9, 1056, doi: 10.1038/s41419-018-1074-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin SJ Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 283, 2599–2615, (2016). [DOI] [PubMed] [Google Scholar]
- 109.Arpaia N et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 162, 1078–1089, (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greter M et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Merad M, Ginhoux F & Collin M Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol 8, 935–947, (2008). [DOI] [PubMed] [Google Scholar]
- 112.Igyarto BZ et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ & Visvader JE Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 11, R62, doi: 10.1186/bcr2353 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gouon-Evans V, Rothenberg ME & Pollard JW Postnatal mammary gland development requires macrophages and eosinophils. Development 127, 2269–2282 (2000). [DOI] [PubMed] [Google Scholar]
- 115.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA & Henson PM Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod 78, 586–594, (2008). [DOI] [PubMed] [Google Scholar]
- 116.O’Brien J, Martinson H, Durand-Rougely C & Schedin P Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 139, 269–275, (2012). [DOI] [PubMed] [Google Scholar]
- 117.Minutti CM et al. Local amplifiers of IL-4Ralpha-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sommerfeld SD et al. Interleukin-36gamma-producing macrophages drive IL-17-mediated fibrosis. Sci. Immunol 4, doi: 10.1126/sciimmunol.aax4783 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gosselin D et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakai M et al. Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity 51, 655–670 e658, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohen M et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 175, 1031–1044 e1018, (2018). [DOI] [PubMed] [Google Scholar]
- 122.Buechler MB et al. A Stromal Niche Defined by Expression of the Transcription Factor WT1 Mediates Programming and Homeostasis of Cavity-Resident Macrophages. Immunity 51, 119–130 e115, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Svedberg FR et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat. Immunol 20, 571–580, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Beek AA, Van den Bossche J, Mastroberardino PG, de Winther MPJ & Leenen PJM Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 40, 113–127, (2019). [DOI] [PubMed] [Google Scholar]
- 125.Frisch BJ et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B. JCI Insight 5, doi: 10.1172/jci.insight.124213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keenan CR & Allan RS Epigenomic drivers of immune dysfunction in aging. Aging Cell 18, e12878, doi: 10.1111/acel.12878 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerlach BD et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ., doi: 10.1038/s41418-019-0370-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Newton K et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574, 428–431, (2019). [DOI] [PubMed] [Google Scholar]
- 129.Riegler AN, Brissac T, Gonzalez-Juarbe N & Orihuela CJ Necroptotic Cell Death Promotes Adaptive Immunity Against Colonizing Pneumococci. Front. Immunol 10, 615, doi: 10.3389/fimmu.2019.00615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cho YS et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Molnar T et al. Current translational potential and underlying molecular mechanisms of necroptosis. Cell. Death Dis 10, 860, doi: 10.1038/s41419-019-2094-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li S, Ning LG, Lou XH & Xu GQ Necroptosis in inflammatory bowel disease and other intestinal diseases. World J. Clin. Cases 6, 745–752, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hermetet F et al. Efferocytosis of apoptotic human papillomavirus-positive cervical cancer cells by human primary fibroblasts. Biol. Cell 108, 189–204, (2016). [DOI] [PubMed] [Google Scholar]
- 134.Roberts EW et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 30, 324–336, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pober JS et al. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature 305, 726–729, (1983). [DOI] [PubMed] [Google Scholar]
- 136.Boots AM, Wimmers-Bertens AJ & Rijnders AW Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology 82, 268–274 (1994). [PMC free article] [PubMed] [Google Scholar]
- 137.Rieder F, Brenmoehl J, Leeb S, Scholmerich J & Rogler G Wound healing and fibrosis in intestinal disease. Gut 56, 130–139, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martinez FJ et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 3, 17074, doi: 10.1038/nrdp.2017.74 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Hernandez-Gea V & Friedman SL Pathogenesis of liver fibrosis. Annu. Rev. Pathol 6, 425–456, (2011). [DOI] [PubMed] [Google Scholar]
- 140.Rowe SM, Miller S & Sorscher EJ Cystic fibrosis. N. Engl. J. Med 352, 1992–2001, (2005). [DOI] [PubMed] [Google Scholar]
- 141.Ratjen F & Doring G Cystic fibrosis. Lancet 361, 681–689, (2003). [DOI] [PubMed] [Google Scholar]
- 142.Elraiyah T et al. A systematic review and meta-analysis of debridement methods for chronic diabetic foot ulcers. J. Vasc. Surg 63, 37S–45S e31–32, doi: 10.1016/j.jvs.2015.10.002 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Cornell RS, Meyr AJ, Steinberg JS & Attinger CE Debridement of the noninfected wound. J. Vasc. Surg 52, 31S–36S, doi: 10.1016/j.jvs.2010.06.006 (2010). [DOI] [PubMed] [Google Scholar]
- 144.Magnus T, Chan A, Linker RA, Toyka KV & Gold R Astrocytes are less efficient in the removal of apoptotic lymphocytes than microglia cells: implications for the role of glial cells in the inflamed central nervous system. J. Neuropathol. Exp. Neurol 61, 760–766, (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.