Figure 1: The sequence of IT->TT suppresses the growth of NRAS-mutant (SW1) and BRAF-mutant (SM1) tumors and limits relapse.
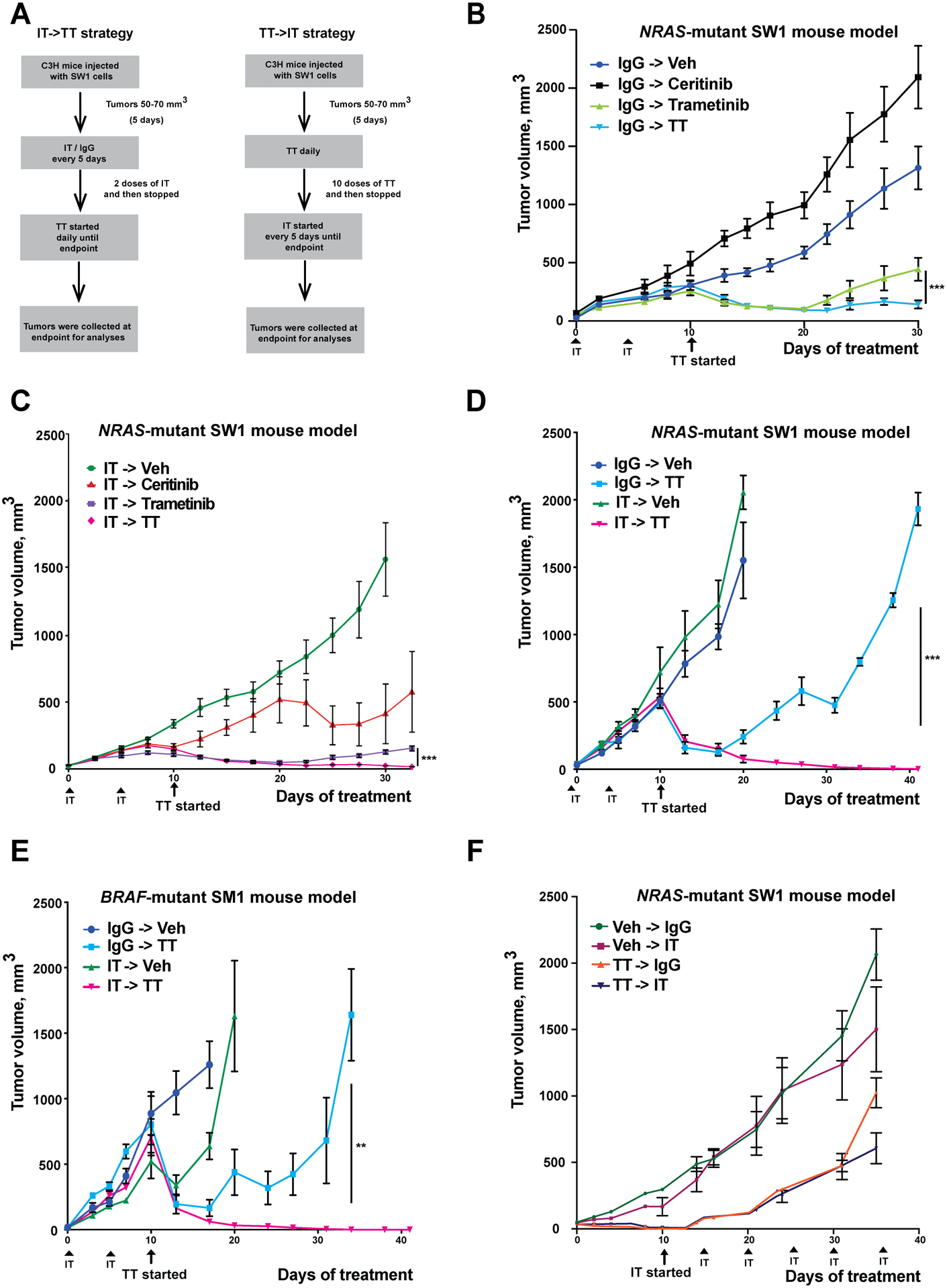
A) Dosing schema for the IT->TT and TT->IT sequences. B) TT alone causes tumor suppression up to day 30 of treatment. Mice received two doses of IgG control (200 μg/100 μl) followed by vehicle, ceritinib (25 mg/kg), trametinib (1 mg/kg) or a combination of ceritinib and trametinib for 30 days. C) Treatment with IT->TT yields durable anti-tumor responses. Mice received two doses of anti-PD1 (200 μg/100 μl) followed by vehicle, ceritinib, trametinib or ceritinib and trametinib combination therapy for 30 days. D) IT->TT sequence leads to long-term anti-tumor responses. Mice were treated with IgG or IT followed by vehicle or combination TT therapy for a period of 41 days. E) Treatment with IT->TT in BRAF-mutant melanoma mouse model system (SM1) shows anti-tumor responses without relapse. Mice were treated as per the SW1 melanoma in C) for 41 days. F) The reverse sequence TT->IT was associated with less tumor suppression in SW1 NRAS-mutant melanoma. Mice received ten doses of vehicle or a combination of ceritinib and trametinib everyday followed by six doses of anti-PD1 or IgG control (200 μg/100 μl) every 5 days for a period of 35 days. The results were represented as average ± SEM. For all panels, 8 mice per group were used, except for Figures 1E,F where 5 mice per group were used. Statistical significance was assessed with one-way ANOVA test (**, 0.0001 ≤ p ≤ 0.001 and ***, p ≤ 0.0001).
