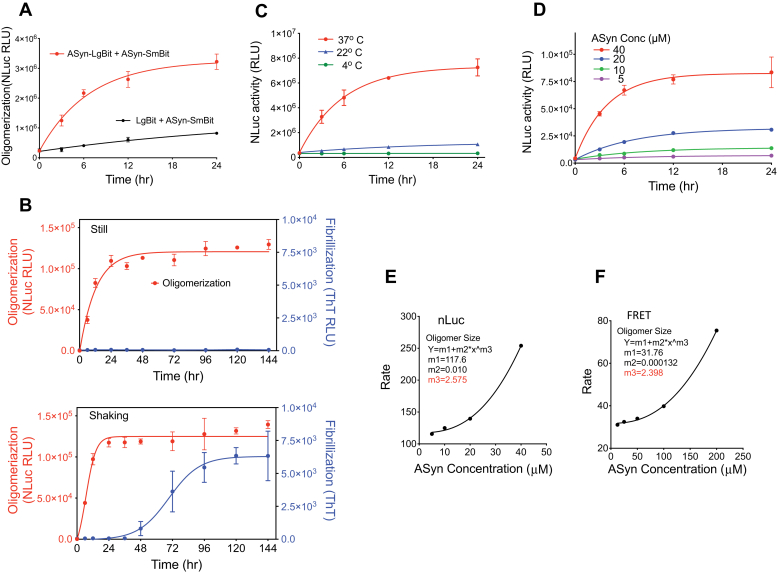
Figure 1.
ASyn biochemical oligomerization assays.A, split nLuc-tagged ASyn biochemical oligomerization assay development. LgBiT protein alone (black) or ASyn protein tagged with LgBit (ASyn-LgBiT, red) is mixed with ASyn protein tagged with SmBit (ASyn-SmBiT) at 10 μM each, incubated without shaking at 37 °C, and assayed for nLuc activity at various times. Oligomerization is detected by complementation of the split tags attached to ASyn. B, in total, 10 μM each of ASyn-LgBiT and ASyn-SmBiT is incubated in PBS under still (top panel) or shaking (bottom panel) conditions and analyzed for either ASyn oligomerization using the nLuc assay (red) or fibrillization via ThT fluorescence (blue). Oligomerization did not require shaking, whereas fibrillization did. C, temperature dependence of split nLuc-tagged ASyn oligomerization assay. Tagged ASyn was incubated at various temperatures and nLuc activity measured. 37 °C was required for robust oligomer formation. D, various concentrations of ASyn-LgBiT and ASyn-SmBiT were assayed for ASyn oligomerization using the nLuc assay. Oligomerization is dependent on ASyn concentration. E, initial rates of ASyn oligomerization in the nLuc assay, calculated from the data in D, are plotted against ASyn concentration and analyzed by the formula Y = m1 + m2∗xˆm3 to derive a minimal size of detected ASyn oligomer (m3) of 2.6 ASyn molecules. F, initial rates of ASyn oligomerization in the FRET assay, calculated from the data in Fig. S4, are plotted against ASyn concentration. Analysis as in E derived a minimal size of detected ASyn oligomer (m3) of 2.4 ASyn molecules. In all graphs the symbols show mean and bars show ±s.d., n = 3.