Figure 1. Rpl11 haploinsufficiency results in a CFU-E/proerythroblast block.
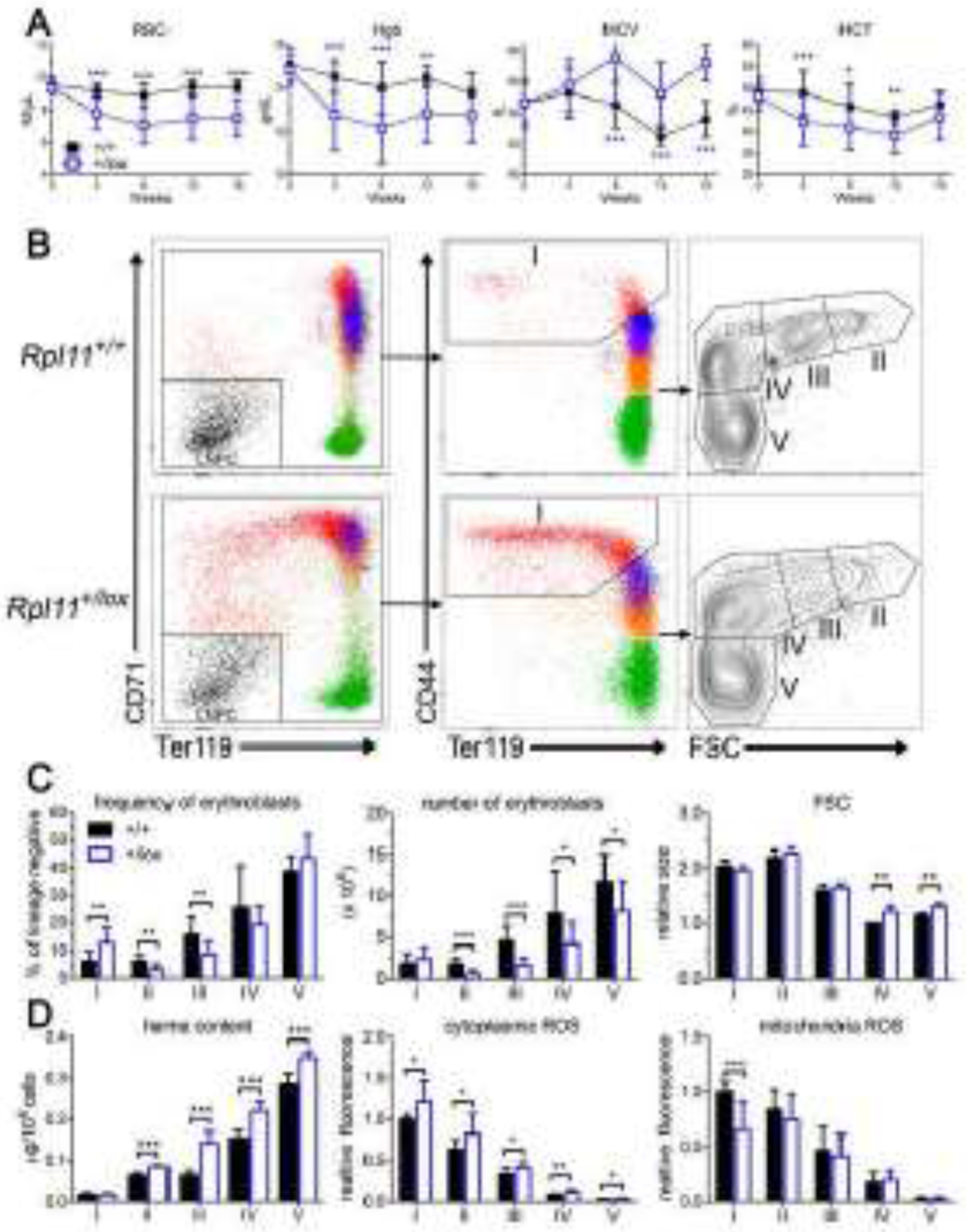
A. CBC analysis of control (+/+) and Rpl11 haploinsufficient (+/lox) mice shows macrocytic anemia develops by 8 weeks after deletion. B. Representative flow cytometry analysis of control (+/+) or Rpl11 haploinsufficient (+/lox) marrow erythroblasts. Lineage negative whole marrow was gated and analyzed as before [19] to identify the lineage negative precursor cells (LNPC) and erythroblast populations I-V which include (I) BFU-E, CFU-E, proerythroblasts, (II) basophilic, (III) polychromatic, (IV) orthochromatic, and (V) reticulocytes and mature RBC. C. Analysis of erythroid precursor cell frequency and numbers from control (+/+) and Rpl11 haploinsufficient (+/lox) mice revealed a CFU-E/proerythroblast block in differentiation with increased cell size (MCV) of populations IV-V. D. Rpl11 haploinsufficient erythroid precursor cells have increased heme content in populations II-V, and increased cytoplasmic ROS but not mitochondrial ROS, similar to results from Flvcr1-deleted mice [19]. Data from 12 control and 12 RPL11 haploinsufficient mice except heme content and erythroblast size is from 4 control and 4 RPL11 haploinsufficient mice. Data is presented as mean ± SD. *: P ≤ 0.05; **: P ≤ 0.01; ***: P ≤ 0.001.
