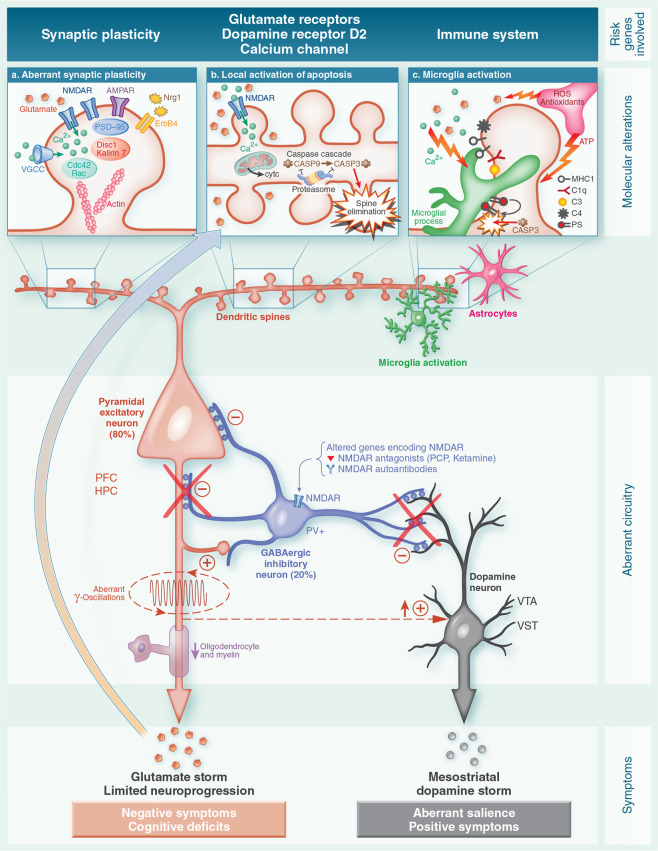
Fig. 1. From risk genes to clinical symptoms.
Highly simplified diagram summarizing the hypothesized pathways toward dendritic apoptosis underlying the overpruning of dendritic spines occurring in late adolescence and early adulthood in individuals with schizophrenia. The central part of the diagram depicts the alterations reported in brain cortical circuits in individuals with schizophrenia and in animal models of this illness (Figure modified from Marín)44. Although not represented here separately, it should be noted that inhibitory interneurons (central neuron) are located either in the cerebral cortex (prefrontal cortex –PFC- and hippocampus –HPC-) or in the ventrotegmental area (VTA) and ventral striatum (VST) dopaminergic areas. The altered encoding (among other possible causes) of glutamate N-methyl-D-aspartate receptors (NMDAR) could produce a dysfunction of inhibitory GABAergic interneurons expressing parvalbumin (PV+) resulting in disinhibition of excitatory pyramidal cells (left neuron). Note that there are two translational models supporting this view: anti-NMDAR autoimmune encephalitis and the administration of NMDAR antagonists such as phencyclidine (PCP) and ketamine. The imbalance of excitatory and inhibitory neurons are proposed to lead to aberrant gamma oscillations thereby contributing to the cognitive dysfunction and primary negative symptoms. Abnormalities of oligodendrocytes and myelin have also been described. The dysfunction of GABAergic inhibitory interneurons could produce a regionally-located significant release of glutamate (glutamate storm) by excitatory glutamatergic cortical pyramidal neurons as well as a subcortical hyperdopaminergic state (dopamine storm). The elevations in extracellular glutamate might act as a pathogenic driver in the brain triggering apoptosis and limited neuroprogression. On the other hand, the disinhibition of the mesostriatal dopaminergic neurotransmission (right neuron) could cause aberrant salience and either premorbid sub-threshold psychotic symptoms or, if sustained, the full blown-onset of a first psychotic episode. a Aberrant synaptic plasticity. Altered genes encoding the fine-tuning of the glutamate synapse which are crucial for spine plasticity and maintenance: NMDAR (GRIN2A), α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors (GRIA1), neuroregulin 1 (NRG1) and its receptor ErbB4, cytoskeletal proteins of the dendritic spines (Actin, ARC complex, RHO, CDC42, Rac, PSD95, DISC1, Kalirin-7). Their dysfunction may contribute to exaggerated spine loss, leading to brain misconnections and synaptic inefficiency (Figure modified from 0wen and colleagues)3. b Local activation of dendritic apoptosis. In critical periods, the glutamate storm and calcium overload via NMDAR could trigger local activation of the dendritic mitochondrial apoptosis pathway and caspase-3 cascade leading to the overpruning of spines and dendrites. Interestingly, altered encoding of voltage-gated calcium channels (VGCC) gene (CACNA1C) has been reported and could contribute to making neurons susceptible to calcium overload. As depicted in the figure, proteasomes act as brakes preventing the spread of the apoptosis mechanism to the cell body, thus avoiding cell death (Figure modified from Ertürk and colleagues)87. c Microglia activation. Apoptotic dendrites and other molecules generate “find me” signals and “eat me” signals (e.g.,phosphatidylserine-PS-) to attract microglia contributing to the phagocytosis of synapses. Some candidate molecules such as proteins of the major histocompatibility complex (MHC) class 1 and complement cascade proteins (C1-C3, and, more recently, C4) have also been implicated. Thus, microglia could contribute to the overpruning of dendritic spines in critical periods of neurodevelopment (Figure modified from Miyamoto and colleagues)97.