Abstract
Rodent research suggests that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and the resulting cortisol stress response can alter the structure of the hippocampus and amygdala. Because early-life changes in brain structure can produce later functional impairment and potentially increase risk for psychiatric disorder, it is critical to understand the relationship between the cortisol stress response and brain structure in early childhood. However, no study to date has characterized the concurrent association between cortisol stress response and hippocampal and amygdala volume in young children. In the present study, 42 young children (Mage = 5.97, SD = 0.76), completed a frustration task and cortisol response to stress was measured. Children also underwent magnetic resonance imaging (MRI), providing structural scans from which their hippocampal and amygdala volumes were extracted. Greater cortisol stress response was associated with reduced right amygdala volume, controlling for whole brain volume, age, sex, and number of cortisol samples. There were no significant associations between cortisol stress response and bilateral hippocampus or left amygdala volumes. The association between right amygdala volume and cortisol stress response raises the non-mutually exclusive possibilities that the function of the HPA axis may shape amygdala structure and/or that amygdala structure may shape HPA axis function. As both cortisol stress response and amygdala volume have been associated with risk for psychopathology, it is possible that the relationship between cortisol stress response and amygdala volume is part of a broader pathway contributing to psychiatric risk.
Keywords: Cortisol stress response, Amygdala, Hippocampus, Structural MRI, Early childhood
1. Introduction
The relationship between developing brain structure and the cortisol stress response remains poorly understood. This is a noteworthy gap in the literature given that rodent studies suggest that a dysregulated cortisol stress response produces alterations in the structure of the hippocampus and amygdala (Magarinos and McEwen, 1995; Sapolsky et al., 1986). Both structures in the subcortex of the brain, the hippocampus and amygdala serve critical functions. Specifically, the hippocampus is involved in learning and memory (Karlsgodt et al., 2005), while the amygdala is involved in emotion processing and salience detection (Phelps, 2006). Because early childhood is a period of enormous neurobiological growth (Giedd et al., 1999), it is possible that early alterations to the structure of the hippocampus and amygdala could disrupt their function, impairing emotional and cognitive development. Indeed, a correlation between heightened cortisol stress response and heightened psychiatric symptoms has been observed in children (Gaffrey et al., 2018), and, similarly, alterations in hippocampal (Barch et al., 2019) and amygdala structure (Tottenham et al., 2010) have also been observed in children with psychiatric symptoms. These findings suggest that the relationship between cortisol stress response and brain structure in childhood may contribute to the development of psychopathology. However, before it is possible to intervene to prevent any hypothesized disruptions to early childhood brain or behavioral development, a key first step is to better understand the relationship between the cortisol stress response and the structure of the hippocampus and amygdala in early childhood. In the present study, we examine the concurrent relationship between variation in the cortisol stress response and hippocampal and amygdala volume in preschool age children.
The hypothalamic-pituitary-adrenal (HPA) axis describes the complex inter-relationship between the hypothalamus, pituitary gland, and adrenal gland. When the hypothalamus detects a stressor in the environment, it sends neural signals to the pituitary gland, which, in turn, signals to the adrenal gland to release cortisol, a stress hormone. This process initiates the cortisol stress response, an increase in cortisol that peaks roughly 30 min after the onset of an acute stressor and returns to baseline levels approximately 1 h later (Seltzer et al., 2010; Send et al., 2019). Release of cortisol in response to a stressor mobilizes energy resources, increases the inflammatory response, and suppresses the immune system (Herman et al., 2011). These functions are helpful in coping with an immediate, short-term threat. However, they can also be damaging when the HPA axis is hyperactive and too much cortisol is released: cortisol is able to cross the blood-brain barrier (Mora et al., 2012), and its presence can promote toxicity and cell death in the brain (Behl et al., 1997; Sapolsky et al., 1986).
In rodents, hypersecretion of corticosterone—the rodent equivalent of cortisol—has been linked to neuroanatomical changes, particularly in the hippocampus and amygdala. Specifically, in the rodent hippocampus and amygdala, neuronal death (Ding et al., 2010; Sapolsky et al., 1986) and dendritic retraction (Conrad, 2006; Magariños et al., 1998; Vyas et al., 2002) have been observed following major stress and corticosterone administration. These findings generally suggest that shrinkage of the hippocampus and amygdala follows corticosterone hypersecretion, although the directionality of these findings is not always consistent. (For example, see Mitra and Sapolsky (2008) for a positive correlation between corticosterone administration and dendritic branching in the amygdala). Moreover, rodent studies suggest that the developing rodent brain is particularly susceptible to the effects of stress and stress hormones. In rodents, heightened corticosterone in early life can have a larger impact on brain structure than elevations in corticosterone experienced later on (Sousa et al., 1998).
While these rodent studies suggest that corticosterone hypersecretion impacts the developing brain's subcortical structure, the literature describing this relationship in human children is sparse. To date, very few studies have measured the relationship between cortisol stress response and hippocampal and amygdala structure in children, although a larger number of studies have examined the relationship between hippocampal and amygdala structure and other measures of cortisol. In this broader work, findings have been mixed. Some authors find that elevations in cortisol are associated with larger amygdala volumes (Buss et al., 2012), some smaller (Pagliaccio et al., 2014). Most authors find a relationship between a smaller hippocampal volume and elevations in cortisol, but some have suggested this relationship is only present for specific subregions of the hippocampus (Blankenship et al., 2019; Merz et al., 2019).
These discrepancies may be due to the fact that not all of these studies have measured cortisol via the cortisol stress response. Cortisol can be measured as the cortisol stress response, but researchers may also measure overall levels of cortisol by sampling concentrations of the hormone in hair or by taking saliva samples at rest. These measures are often considered interchangeable; yet a recent study which measured cortisol stress response, hair cortisol, and resting salivary cortisol levels in 400 youths found only small correlations among these measures (Malanchini et al., 2020). In addition, if a dysregulated cortisol stress response alters brain structure in early life, ,then it is particularly important to understand how this specific measure of cortisol relates to brain structure during early childhood. To date, two studies have specifically examined the relationship between cortisol stress response and subcortical brain structure in children: Blankenship et al. (2019) and Pagliaccio et al. (2014). The former found that a heightened cortisol stress response in early childhood (ages 3–5.96 years) predicted smaller hippocampal volumes in later childhood (ages 5.57–10 years), but only in the hippocampal body; in contrast, the latter reported that cortisol stress response in early childhood (ages 3–5 years) predicted smaller volumes in the bilateral amygdala and the left hippocampus in later childhood (ages 7–12 years). However, critically, both of these studies measured cortisol stress response in early childhood and then measured brain structure at a later time point (Blankenship et al., 2019; Pagliaccio et al., 2014). The concurrent relationship between cortisol stress response and hippocampal and amygdala structure during early childhood—when the brain is likely most susceptible to the effects of cortisol—has not yet been explored (Gunnar, 2017).
In the present study, we begin to address some of the limitations in the existing literature by concurrently measuring cortisol stress response and hippocampus and amygdala structure in a sample of preschool age children. In line with the research reviewed above, we hypothesized that a heightened cortisol stress response would correlate with alterations in hippocampal and amygdala volume. We did not hypothesize that cortisol stress response would correlate with larger or smaller hippocampal or amygdala volume given the mixed nature of previous findings (Blankenship et al., 2019; Pagliaccio et al., 2014).
2. Material and methods
2.1. Participants
Seventy-three children completed all parts of a behavioral assessment and a magnetic resonance imaging (MRI) session. During recruitment, parents completed a brief screening questionnaire over the phone. Children with neurological disorder, autism spectrum disorder, developmental delays, MRI contraindications, use of psychiatric medication, and/or who were born prematurely (e.g., <36 weeks of gestation) were excluded from study participation. Parents were also asked about their children's depressive symptoms using the short version of the Preschool Feelings Checklist (PFC; Luby et al., 1999). Depressive symptoms were measured during recruitment because the children in this study were part of a larger study examining the development of depressive symptoms beginning at preschool age (see Gaffrey et al. (2018)). Children were recruited from day care centers, pediatrician's offices, and the community at-large. 33.3% of children in the present study sample had elevated depressive symptoms. Elevated depressive symptoms were defined as a score of 3 or higher on the short version of the PFC. A score of 3 indicates the presence of 3 or more behaviors associated with core depressive symptoms (e.g., change in appetite, pretend play about scary or sad things). A detailed description of this measure can be found in the Supplement. Analyses are conducted with and without controlling for depressive symptoms.
All children participated with their biological mother. Mothers signed a written consent form, and children provided verbal assent. Families were compensated for their participation. During the behavioral assessment, mothers completed questionnaires regarding their and their child's mental health, physical health, behavior, and life experiences. Children completed behavioral tasks, including a frustration task and cortisol sampling procedure detailed below. The MRI session occurred approximately 7–10 days after the behavioral assessment (M = 8.52 days, SD = 6.18). The Washington University in St. Louis Institutional Review Board approved the study. All research was carried out in accordance with the Declaration of Helsinki.
46 of the 73 children who completed both study sessions produced very high-quality imaging data. Prior work demonstrates that MRI scans with motion artifacts may bias results and that obtaining low movement MRI data with developmental populations or populations with psychiatric symptoms—both of which describe the current sample—is highly challenging (Backhausen et al., 2016). 63 of the 73 children provided high-quality salivary cortisol samples. These two groups overlapped to produce a final sample of 42 children who generated both high-quality salivary cortisol samples and high-quality structural brain images.
The final sample of N = 42 children (Mage = 5.97 years, 38% male) was 26% African American, 69% White, and 5% Multiracial. Thirty-three percent displayed elevated depressive symptoms on the short version of the PFC when screened during recruitment. Using a two sample t-test, there was no difference in short version PFC scores during screening (t(71) = −0.03, p = .97) or scale version PFC scores at the assessment (t(71) = −0.45, p = .66) between children in the present sample of N = 42 and those excluded due to poor-quality imaging and cortisol data. There was also no difference in cortisol values between children in the present sample and those excluded due to poor-quality imaging data (t(61) = −0.42, p = .68). Statistics describing the sample can be found in Table 1.
Table 1.
Sample characteristics.
| Characteristic | Percentage |
|---|---|
| Sex (M/F) | 38% M, 62% F |
| Race (W, AA, MR) | 69% W, 26% AA, 5% MR |
| Short PFC (High, Low) | 33% High, 66.67% Low |
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 5.97 | 0.76 | 4.31–7.00 |
| Scale PFC | 15.31 | 11.04 | 1–47 |
| Life Events Checklist | 8.69 | 10.36 | 0–41 |
| Cortisol (AUCg) | 36.41 | 6.30 | 22.81–53.59 |
| Left Amygdala Volume (mm3) | 2022.01 | 171.13 | 1701.00–2399.50 |
| Right Amygdala Volume (mm3) | 2085.68 | 155.90 | 1740.60–2526.90 |
| Left Hippocampus Volume (mm3) | 4955.67 | 366.37 | 4173.90–6109.80 |
| Right Hippocampus Volume (mm3) | 5048.02 | 393.27 | 4321.10–5974.70 |
| Whole Brain Volume (mm3) | 1,464,959 | 39,259.10 | 1,391,717–1,557,453 |
Note. M = Male, F = Female. W = White, AA = African American, MR = Multi-racial. PFC = Preschool Feelings Checklist. AUCg = Area Under the Curve with respect to ground.
2.2. Frustration task, cortisol collection, and cortisol analysis
Children completed a well-validated frustration task (Kryski et al., 2011) previously shown to elicit a cortisol stress response in young children (Send et al., 2019). At the start of the task, children were presented with a large board covered in pictures of bumblebees and frogs, a basket containing blue and red Velcro buttons, and a small stoplight (see Supplemental Fig. 1). Children were told that they could earn a prize if they matched the red buttons to the frogs and the blue buttons to the bumblebees within a specific time. The stoplight would signal how much time remained. A green light indicated plenty of time remaining, a yellow light indicated that only a small amount of time remained, and a red light indicated that no time remained. However, the experimenter always triggered the red light before the child completed the task, so that the child never finished the task within the allotted time. Each child attempted three rounds of the task. Immediately after the last round of the task, children were informed that the stoplight was broken. Their matching abilities were praised, and they were offered a prize.
After completing 30 min of neutral activities (e.g., coloring and watching a neutral nature video) and prior to completing the frustration task, a baseline salivary cortisol sample was obtained from each child. Additional saliva samples were collected at 0, 10, 20, 30, 40, and 50 min after the frustration task. These times are relative to the child's completion of the frustration task (e.g., time 0 represents a sample taken as soon as the task was completed). The child and experimenter completed neutral activities while the post-task saliva samples were collected. The sampling procedure began between 10:15 a.m. and 2 p.m. to minimize diurnal variation in cortisol (Kudielka et al., 2004).
Salivary cortisol samples were measured (ng/mL) in duplicate using a commercially available enzyme-linked immunosorbent assay kit according to manufacturer instructions (ELISA; DRG International kit SLV-2930; Springfield, New Jersey USA). Intra- and inter-assay coefficients of variation were 3.80% and 6.85%, respectively. Samples producing unreliable measures (i.e., intra-assay CVs 20%) even after being re-assayed in duplicate were excluded.
A participant had to have the first or second sample and at least 5 of the 7 collected saliva samples to be included in analyses. 38 of the 42 participants had all 7 samples. Salivary stress-induced cortisol response was calculated as the Area Under the Curve with respect to ground (AUCg), which captures the full cortisol response to stress by including both circulating cortisol and the increase in cortisol in response to acute stress (Pruessner et al., 2003). The cortisol assay procedure, quality control, and quantification details are described in the Supplement.
2.3. MRI imaging
2.3.1. Structural MRI acquisition, quality control, and processing
To help ensure child comfort and familiarity with the scan environment, all children completed a mock scan prior to the MRI. Please see the Supplement for more details on this procedure. During the scan, 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) images were acquired using the following parameters: TR = 2400 ms, TE = 3.16 ms, flip angle = 8°, slab = 176 mm, 176 slices, matrix size = 256 × 256, field of view (FOV) = 256 mm, voxel size = 1 × 1 × 1 mm, sagittal plane acquisition. These data were acquired on a Siemens Tim Trio 3T scanner (Siemens Healthcare, Erlangen, Germany).
T1-weighted images were reviewed slice-by-slice using FreeSurfer's image viewing software FreeView (Fischl and Dale, 2000). The quality of each image was rated using the qualitative rating system validated by Backhausen et al. (2016). Specifically, each image was rated on a scale of good, moderate, or bad in the following four categories: overall image sharpness, ringing, clarity of subcortical structures, and clarity of grey/white matter boundaries. Quality ratings of images were performed by C.H.F. and were reviewed by M.S.G. No image with a rating of “bad” in any category was included in the present study. C.H.F. and M.S.G. were blinded to children's cortisol values when reviewing the structural images.
T1-weighted images were processed using the recon-all command of FreeSurfer 6.0 (Fischl and Dale, 2000). Recon-all completes surface-based and volume-based image processing. In FreeSurfer, images are intensity normalized, skull stripped, segmented into grey and white matter, and subcortical volumes are automatically labeled. White matter and pial surfaces are created by following the intensity gradient, and the distinction between the surfaces is placed at light/dark transitions. A detailed description of this processing can be found elsewhere (Fischl, 2012; Fischl and Dale, 2000). Before being processed in FreeSurfer, images were transformed to a common template space using a transformation (Talairach and Tournoux, 1988) that has been validated for use with young children (Ghosh et al., 2010).
Prior work demonstrates that FreeSurfer's calculations of subcortical volumes correlate highly with measurements generated manually in tracing studies (Morey et al., 2009). Further, use of FreeSurfer's volumetric processing stream has been validated for use with young children (Schoemaker et al., 2016) and is regularly used in work examining the relationship between hippocampal/amygdala volume and behavior in developmental populations (Barch et al., 2019).
Following processing in recon-all, all images were visually inspected again. When minor errors were detected in the surfaces (e.g., pial surface capturing skull or dura mater), manual edits were applied. Subcortical structures were not edited. Our reasons for not editing subcortical structures were twofold: first, following review of the segmentations, we did not observe any major problems with the segmentation of the hippocampus or amygdala; second, the makers of FreeSurfer advise FreeSurfer users to avoid editing subcortical volumes when possible, since these changes can sometimes cause even greater problems. For example, see: http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingDataV6.0.
2.3.2. Structural MRI analysis
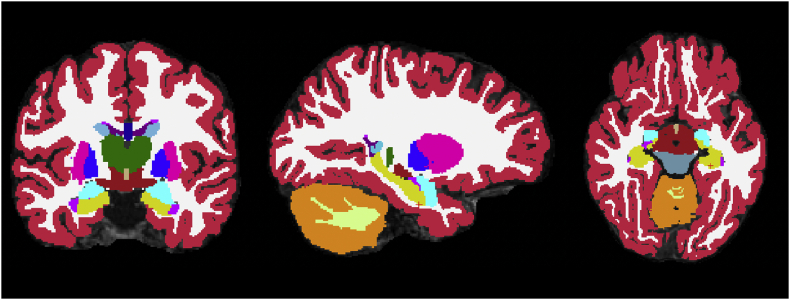
Volumetric measurements of the amygdala, hippocampus, and whole brain were obtained using the asegstats2table command native to FreeSurfer 6.0. (See Fig. 1). This command reports the volumes of all of the structures labeled in the aseg.mgz file generated by recon-all. All statistical analyses were performed in R Studio, version 3.5.3, with the exception of outlier detection, which was performed in IBM SPSS 26.0.
Fig. 1.
Example of a participant's volumetric segmentation. The hippocampus is in yellow. The amygdala is in sky blue.
2.4. Child depressive symptom severity
Mothers completed the scale version of the Preschool Feelings Checklist (PFC–S; Luby et al., 1999) about their child. This measure is a longer, more detailed version of the short version of the Preschool Feelings Checklist that was completed during recruitment. The scale version of the PFC is designed to assess a child's depressive symptoms and the severity of these symptoms in a more detailed manner than the short version of the PFC. Neither the scale version nor the short version may establish the presence or absence of a DSM-5 depression diagnosis (American Psychiatric Association, 2013). The scale version of the PFC contains 23 items, with items such as: “My child pretends or make believes about scary or sad things.”; “My child blames himself for things.” Each item is rated on a 0–4 scale, with 0 = Never and 4 = Most of the Time. Higher scores indicate greater depressive symptom severity. Prior work demonstrates that the PFC-S reliably assesses child depressive symptom severity (Luby et al., 2004). This measure was intended as a covariate in analyses.
2.5. Life Events Checklist
Mothers completed the Life Events Checklist (Johnson and McCutcheon, 1980), a well-validated measure that asks parents about the occurrence and impact of stressful events in their child's life. For example, items include— “parents divorced”; “new brother or sister”—events that are directly relevant to young children. Parents indicate with a 0 or 1 if the event has ever occurred in the child's lifetime or in the last 6 months. For events that have happened, parents indicate if they believe the impact of the event on the child was positive or negative, and they rate the impact of the event on the child using a 1–4 scale (1 = No effect, 2 = Some effect, 3 = Moderate effect, 4 = Great effect). While events are rated on a 1–4 scale, it is possible to obtain a score of 0, if no negative events ever occurred. Impact scores are summed, producing four scores: a positive 6-month impact score, a negative 6-month impact score, a positive lifetime impact score, and a negative lifetime impact score. These reflect, respectively, the impact of positive and negative stressful life events that have occurred within the past 6 months and over the course of the child's lifetime. In our analyses, we employed the negative lifetime impact score because, while negative life events are linked to increased stress, positive life events are generally considered to buffer against stress (Blonski et al., 2016). This measure was intended as a covariate in analyses.
2.6. Analysis plan
Based on our a priori hypotheses, we planned to conduct 4 separate linear regressions to examine the relationship between subcortical structure and cortisol stress response. Right amygdala volume, left amygdala volume, right hippocampal volume, and left hippocampal volume would each be separately regressed onto cortisol stress response values (AUCg). We elected to examine each hemisphere separately because previous research with adults (Barry et al., 2017) and adolescents (Klimes-Dougan et al., 2014) suggests that the relationship between cortisol stress response and subcortical structure may vary by hemisphere. Further, age, sex, and whole brain volume may all influence subcortical volume (Giedd et al., 1999), so these variables were intended to be included as covariates. Additionally, while most children had all 7 cortisol samples (90%), some did not, prompting us to include total number of obtained samples as a covariate in the four primary regressions of interest.
Further, although time of day was accounted for in the experimental design, we also planned to conduct a supplementary analysis to confirm that minor variation within the 3.5-h time frame during which all samples were collected did not impact the results. To do this, we planned to conduct a separate regression controlling for time of day. Additionally, it has been previously observed that children with psychiatric symptoms, such as symptoms of depression (Luby et al., 2003) or anxiety (Kryski et al., 2013), may display a heightened cortisol stress response, while—in contrast—children with histories of early life stress may show a blunted cortisol stress response (Koss et al., 2016). For this reason, two additional regressions were planned, one controlling for stressful, negative life events, and another controlling for depressive symptoms. The sample was not enriched for stressful life events, but because the sample here is a subsample of a larger study examining development of depressive symptoms in preschoolers, one-third of the children in the present sample displayed elevated depressive symptoms.
3. Results
3.1. Mahalanobis D2
Prior to conducting all analyses, we calculated Mahalanobis D2 for each analysis to identify potential multivariate outliers. No outliers (p < .001) were identified.
3.2. Cortisol stress response and subcortical brain volumes
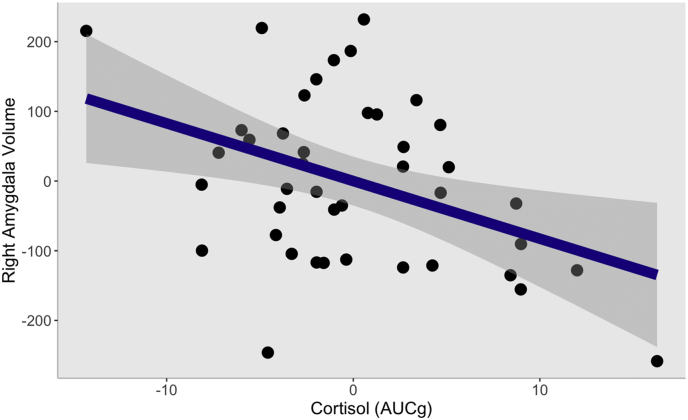
Relationships between AUCg and left hippocampal volume, left amygdala volume, and right hippocampal volume were not significant. However, there was a statistically significant relationship between AUCg and right amygdala volume. Using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995), this result survived correction for multiple comparisons, p = .04. See Table 2a, Table 2b, Table 2c, Table 2dd for statistics. Fig. 2 displays a graphical depiction of the relationship between right amygdala volume and AUCg. Additionally, we also ran a model in which we included the negative impact of stressful life events as a covariate, and a separate model in which we included time of day as a covariate. In each of these models, cortisol stress response remained significantly associated with right amygdala volume (for the regression with stressful life events: B = −8.24, β = -0.33, t(35) = −2.61, p = .01; for the regression with time of day: B = −7.57, β = -0.31, t(35) = −2.31, p = .03). Additionally, a separate regression which included depressive symptoms as a covariate showed that the relationship between AUCg and right amygdala volume was not affected (B = −8.53, β = -0.34, t(35) = −2.51, p = .02).
Table 2a.
Regression of left hippocampal volume onto cortisol stress response.
| B | SE B | β | t | p | |
|---|---|---|---|---|---|
| Constant | 1459.7 | 2312 | n/a | 0.63 | .53 |
| Cortisol (AUCg) | 12.76 | 9.65 | 0.22 | 1.32 | .20 |
| Child age at scan (months) | 4.87 | 7.75 | 0.12 | 0.63 | .53 |
| Child sex | 4.60 | 127.14 | 0.006 | 0.04 | .97 |
| Whole brain volume (mm3) | 0.002 | 0.002 | 0.22 | 1.28 | .21 |
| Number of samples | −57.56 | 119.33 | −0.08 | −0.48 | .63 |
Table 2b.
Regression of left amygdala volume onto cortisol stress response.
| B | SE B | β | t | p | |
|---|---|---|---|---|---|
| Constant | 558.9 | 1068 | n/a | 0.52 | .60 |
| Cortisol (AUCg) | −2.09 | 4.46 | −0.08 | −0.47 | .64 |
| Child age at scan (months) | 3.78 | 3.58 | 0.20 | 1.06 | .30 |
| Child sex | 42.63 | 58.71 | 0.13 | 0.73 | .47 |
| Whole brain volume (mm3) | 0.0007 | 0.0007 | 0.17 | 0.98 | .33 |
| Number of samples | 25.90 | 55.10 | 0.08 | 0.47 | .64 |
Table 2c.
Regression of right hippocampal volume onto cortisol stress response.
| B | SE B | β | t | p | |
|---|---|---|---|---|---|
| Constant | 624.3 | 2435 | n/a | 0.26 | .80 |
| Cortisol (AUCg) | 10.61 | 10.61 | 0.17 | 1.04 | .30 |
| Child age at scan (months) | −5.19 | 8.16 | −0.12 | −0.64 | .53 |
| Child sex | 106.1 | 133.9 | 0.13 | 0.79 | .43 |
| Whole brain volume (mm3) | .003 | .002 | 0.33 | 1.93 | .06 |
| Number of samples | −69.67 | 125.7 | −0.10 | −0.55 | .58 |
Table 2d.
Regression of right amygdala volume onto cortisol stress response.
| B | SE B | β | t | p | |
|---|---|---|---|---|---|
| Constant | −1299 | 747.0 | n/a | −1.74 | .09 |
| Cortisol (AUCg) | −8.28 | 3.12 | −0.33 | −2.65 | .01* |
| Child age at scan (months) | −7.27 | 2.50 | −0.43 | −2.90 | .006** |
| Child sex | −11.62 | 41.1 | −0.04 | −0.28 | .78 |
| Whole brain volume (mm3) | .003 | .0005 | 0.66 | 4.94 | .00002*** |
| Number of samples | 54.30 | 38.55 | 0.18 | 1.41 | .17 |
Note. *p < .05, **p < .01, ***p < .005. B represents the unstandardized regression coefficient. β represents the standardized regression coefficient.
Fig. 2.
Partial regression plot of the relationship between cortisol stress response (AUCg) and right amygdala volume, controlling for whole brain volume, age, sex, and total number of cortisol samples. Grey boundary represents 95% confidence interval. AUCg = Area Under the Curve with respect to ground.
We also noticed that the regression coefficient for whole brain volume in the regression of right amygdala volume onto cortisol stress response was highly significant. This prompted us to investigate the correlations among whole brain volume, right amygdala volume, and left amygdala volume. As anticipated, there was a significant bivariate correlation between right and left amygdala volume, r = 0.44, p = .004. There was also a significant bivariate correlation between whole brain volume and right amygdala volume, r = 0.55, p = .0002. However, there was not a significant correlation between whole brain volume and left amygdala volume, r = 0.20, p = .21. Graphs of these relationships are included in the Supplement. While there were no multivariate outliers according to Mahalanobis D2 calculations, the plot displayed in Supplemental Fig. 2 suggests that a few points shape the left amygdala volume/whole brain volume relationship, preventing these variables from being as correlated as they otherwise might be in a larger sample.
4. Discussion
In rodents, hypersecretion of cortisol can change the structure of the hippocampus and amygdala (Magarinos and McEwen, 1995; Sapolsky et al., 1986). However, to date, only two studies (Blankenship et al., 2019; Pagliaccio et al., 2014) have examined the link between childhood cortisol stress response and subcortical structure, and these studies have examined cortisol stress response in childhood and brain structure years later. The present work is the first to demonstrate a concurrent relationship between HPA axis function (via the cortisol stress response) and hippocampal and amygdala volume in early childhood—when the brain is thought to be most vulnerable to negative effects of stress and stress hormones (Gunnar, 2017). Controlling for number of cortisol samples, age, sex, and whole brain volume, we found that heightened cortisol stress response correlated with reduced volume in the right amygdala, but not the left amygdala or either of the hippocampi. This result also held when controlling for the negative impact of stressful life events and depressive symptoms. While time of day was accounted for by the experimental design, a supplementary analysis that controlled for variation in the 3.5-h window in which all visits took place also yielded the same results. These findings suggest that a heightened cortisol stress response may interfere with the structural development of the right amygdala, possibly via a neurotoxic pathway in which a heightened cortisol stress response promotes cellular damage in the structure (Behl et al., 1997; McEwen, 2005; Sapolsky et al., 1986). Alternatively, these findings could also suggest that a smaller right amygdala volume predisposes children to display a heightened cortisol stress response—an idea that is consistent with the amygdala's involvement in initiating the cortisol stress response.
This finding fits well with the existing work on cortisol stress response and amygdala volume. In line with the present findings, Barry et al. (2017) found that heightened cortisol stress response correlated with a smaller right—but not left—amygdala volume in young adults with postnatally depressed mothers. Similarly, Pagliaccio et al. (2014) found a relationship between preschool cortisol stress response and reduced amygdala volume several years later. Further, Klimes-Dougan et al. (2014) also found that a heightened cortisol stress response was associated with altered volume in the right—but not the left—amygdala in depressed versus healthy teenagers. Interestingly, the result of Klimes-Dougan et al. (2014) only corresponds to that of our sample for their healthy adolescent participants. They found that, for healthy participants, a heightened cortisol response to stress correlated with a smaller right amygdala volume; in contrast, for depressed adolescents, heightened cortisol stress response correlated with a larger right amygdala. This finding essentially corresponds to the present findings in the sense that, in our sample, the relationship between heightened AUCg and smaller right amygdala held when controlling for children's depressive symptoms.
One interpretation of the present findings is that an elevated cortisol stress response might be disruptive to the structural development of the right amygdala. Possibly, a heightened cortisol stress response alters the structure of the right amygdala via a neurotoxic pathway. One possibility is that cortisol hypersecretion may directly impact the structure of the amygdala via cellular toxicity. For example, the presence of glucocorticoids has been demonstrated to be toxic to neurons (Sapolsky et al., 1986) and to amplify the effects of other neurotoxic insults in the brain (Behl et al., 1997). Thus, it could be that cortisol hypersecretion begins a process that directly alters the structure of the amygdala. Another possibility is that cortisol hypersecretion could lead to hyper-reactivity of the amygdala (Dannlowski et al., 2013), which may, over time, produce cellular toxicity and cell death, leading to volumetric shrinkage of the amygdala (Hanson et al., 2015; McEwen, 2005).
Another, less explored possibility is that a smaller right amygdala may contribute to a greater cortisol stress response. Because we measured both cortisol stress response and amygdala volume at the same time point, our findings are also consistent with this hypothesis. The idea that amygdala structure may influence the cortisol stress response stems from research demonstrating that the amygdala helps initiate the cortisol stress response (Herman et al., 2011). In response to an acute stressor, the amygdala relays information about the stressor to the bed nucleus of the stria terminalis, which, in turn, projects to the paraventricular nucleus (PVN) of the hypothalamus. The PVN then triggers the hormonal cascade that results in the release of cortisol. Yet, most research on stress and development does not consider the possibility that amygdala structure may influence cortisol secretion as well as vice versa. To our knowledge, only one study—VanTieghem et al. (2020)—has considered this possibility and attempted to disentangle the effect of cortisol secretion on the amygdala from the effect of the amygdala on cortisol secretion. In this work, the authors examined a large sample of youth who experienced early caregiving adversity and healthy controls at two separate time points. The authors found that amygdala volume at baseline predicted morning cortisol levels at follow up, but that the inverse was not true: cortisol levels at baseline did not predict amygdala volume at follow up. However, the VanTieghem et al. (2020) work did not specifically examine cortisol stress response, instead examining morning and diurnal cortisol at rest.
Additionally, it is also interesting to note that, in our study and in the studies of others, the volume of the right, but not the left, amygdala correlates with a heightened cortisol stress response. Notably, the right amygdala, more so than the left, is implicated in the processing of negative emotion (Lanteaume et al., 2007)—a phenomenon that has been observed even in preschool children (Gaffrey et al., 2013). Because early studies on amygdala function suggested that the amygdala was critically important for threat detection (Adolphs, 2002), some researchers have suggested that a larger amygdala would be maladaptive and cause greater distress due to excessive threat detection (Tottenham et al., 2010). However, research has also found that the amygdala is also involved in emotion processing and salience detection more broadly (Cunningham and Brosch, 2012), potentially suggesting that a smaller amygdala might produce impairments more generally in emotion processing. In line with the idea that a smaller right amygdala contributes to a greater cortisol stress response, it is possible that a smaller right amygdala contributes to less thorough processing of negative stimuli, which, in turn, produces a hyper-reactive cortisol stress response to those stimuli.
Beyond the amygdala, we also hypothesized that we would find a relationship between cortisol stress response and hippocampal volume—a relationship that we did not observe in the present study. Blankenship et al. (2019), Merz et al. (2019), and VanTieghem et al. (2020) have previously detected a relationship between elevated cortisol and reduced hippocampal volume in children. However, notably, Blankenship et al. (2019) and Merz et al. (2019) only detected the influence of cortisol on specific regions of the hippocampal subfields. Methodological difference could explain the discrepancy between the findings of these previous studies and the findings reported here. While we examined the concurrent association between cortisol stress response and hippocampal volume, Blankenship et al. (2019) examined the relationship between cortisol stress response in the preschool period and hippocampal volume later in childhood. Additionally, Merz et al. (2019) examined cortisol found in hair, and VanTieghem et al. (2020) examined morning and diurnal cortisol levels. Alternatively, because this research is the first work to test for a relationship between cortisol stress response and hippocampal volume in preschool age children, another possibility is that there is not yet a relationship between cortisol stress response and hippocampal volume at the early age of the children in our sample. Indeed, some rodent work suggests that stress-related hippocampal shrinkage does not appear until later in life (Andersen and Teicher, 2004; Monroy et al., 2010).
Further, it is important to note that both cortisol stress response and amygdala volume have been linked to psychiatric disorder. Previous researchers have found that a heightened cortisol stress response correlates with greater symptoms of depression (Gaffrey et al., 2018) and anxiety (Kryski et al., 2013) in young children. Similarly, altered amygdala volume has been associated with these same psychiatric disorders (Hamilton et al., 2008; Milham et al., 2005). Our results contribute to this literature. Indeed, in the Supplement, we also conducted exploratory analyses examining the relationship among depressive symptoms, cortisol stress response, and subcortical brain structure. As expected, given that the current study is a subsample from Gaffrey et al. (2018), we found a positive correlation between depressive symptoms and cortisol stress response. However, we did not observe a relationship between depressive symptoms and any subcortical structure tested. Our results could suggest a mechanistic relationship by which cortisol stress response influences both brain structure and behavior, but we shy away from over-interpreting these exploratory results given that only 1/3 of 42 participants (N = 14) displayed elevated depressive symptoms. Longitudinal studies, studies with larger samples, and studies focusing on participants with psychiatric diagnoses are needed to more thoroughly examine the relationship among cortisol stress response, amygdala structure, and depression.
Finally, this study is not without limitations. First, while it is a crucial first step to demonstrate an association between cortisol stress response and subcortical brain structure in preschool age children, our analyses cannot establish any temporal relationships among these variables. That is, because our study is cross-sectional, our reported findings are correlational in nature and cannot inform causation. Future studies should employ prospective longitudinal designs in order to determine the temporal ordering of relationships among the variables studied here. Second, despite our sample size being similar to other studies of young children (Merz et al., 2019), it is, nevertheless, small. A small sample size limits study power and precision of effect size estimates, particularly when small effect sizes are to be expected (Dick et al., 2020). It is also possible that a relationship between cortisol stress response and hippocampal volume was not detected due to the small sample size. Third, we were not able to use data from 21 of the participants who provided high-quality salivary cortisol due to participant motion in the scanner; however, there was no difference in cortisol values between those who scanned and those who did not. Finally, while the racial and ethnic makeup of our sample generally matched the diversity of the community where this study took place, our sample is not representative of the broader United States, as there were no Asian American or Native American families in our sample. Future studies should ensure participation of these groups.
5. Conclusions
This research explored the concurrent association between cortisol stress response and subcortical brain structure in a sample of young children. We found that elevated cortisol stress response was associated with reduced right amygdala volume, controlling for whole brain volume, age, sex, and number of cortisol samples. This result also held when controlling for the negative impact of stressful life events, time of day, and depressive symptoms. By demonstrating a link between cortisol stress response and subcortical brain structure in young children, our findings represent an important first step in understanding the relationship between stress hormones and the structure of the developing brain. This work supports the idea that how children respond to stress could play an important role in their neurodevelopmental trajectory. Further, because both cortisol stress response and amygdala volume have been linked to psychiatric disorder, this work raises the possibility that cortisol hypersecretion and alterations in amygdala structure may represent one step in a pathway that increases risk for psychiatric diagnosis.
Funding source
This work was supported by the National Institute of Mental Health (Grant Nos. K23 MH098176 and R01 MH110488 to MSG) and McDonnell Center for Systems Neuroscience (to MSG). The funding source did not have a role in the study design, data collection, data analysis, data interpretation, or writing of the report.
CRediT authorship contribution statement
Carina H. Fowler: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Ryan Bogdan: Investigation, Formal analysis, Resources, Writing – review & editing. Michael S. Gaffrey: Conceptualization, Funding acquisition, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
None.
Acknowledgements
We would like to thank the children and families who participated in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100329.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. doi: 10.1016/S0959-4388(02)00301-X. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. 2013. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC. [DOI] [Google Scholar]
- Andersen S.L., Teicher M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Backhausen L.L., Herting M.M., Buse J., Roessner V., Smolka M.N., Vetter N.C. Quality control of structural MRI images applied using FreeSurfer-a hands-on workflow to rate motion artifacts. Front. Neurosci. 2016;10:1–10. doi: 10.3389/fnins.2016.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Tillman R., Kelly D., Whalen D., Gilbert K., Luby J.L. Hippocampal volume and depression among young children. Psychiatry Res. Neuroimaging. 2019;288:21–28. doi: 10.1016/j.pscychresns.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry T.J., Murray L., Fearon P., Moutsiana C., Johnstone T., Halligan S.L. Amygdala volume and hypothalamic-pituitary-adrenal axis reactivity to social stress. Psychoneuroendocrinology. 2017;85:96–99. doi: 10.1016/j.psyneuen.2017.07.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C., Lezoualc’h F., Trapp T., Widmann M., Skutella T., Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Blankenship S.L., Chad-Frieman E., Riggins T., Dougherty L.R. Early parenting predicts hippocampal subregion volume via stress reactivity in childhood. Dev. Psychobiol. 2019;61:125–140. doi: 10.1002/dev.21788. [DOI] [PubMed] [Google Scholar]
- Blonski S.C., Conradi H.J., Oldehinkel A.J., Bos E.H., De Jonge P. Associations between negative and positive life events and the course of depression: a detailed repeated-assessments study. J. Nerv. Ment. Dis. 2016;204:175–180. doi: 10.1097/NMD.0000000000000445. [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U.S.A. 2012;109 doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cognit. Neurosci. Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Brosch T. Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr. Dir. Psychol. Sci. 2012;21:54–59. doi: 10.1177/0963721411430832. [DOI] [Google Scholar]
- Dannlowski U., Kugel H., Huber F., Stuhrmann A., Redlich R., Grotegerd D., Dohm K., Sehlmeyer C., Konrad C., Baune B.T., Arolt V., Heindel W., Zwitserlood P., Suslow T. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum. Brain Mapp. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Watts A.L., Heeringa S., Lopez D.A., Bartsch H., Fan C.C., Palmer C., Reuter C., Marshall A., Haist F., Hawes S., Nichols T.E., Barch D.M., Jernigan T.L., Garavan H., Grant S., Pariyadath V., Hoffman E., Neale M., Paulus M.P., Sher K.J., Thompson W.K. Meaningful effects in the adolescent brain cognitive development study. bioRxiv. 2020 doi: 10.1101/2020.09.01.276451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Han F., Shi Y. Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. J. Psychiatr. Res. 2010;44:48–55. doi: 10.1016/j.jpsychires.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Barch D.M., Bogdan R., Farris K., Petersen S.E., Luby J.L. Amygdala reward reactivity mediates the association between preschool stress response and depression severity. Biol. Psychiatr. 2018;83:128–136. doi: 10.1016/j.biopsych.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Barch D.M., Singer J., Shenoy R., Luby J.L. Disrupted amygdala reactivity in depressed 4- to 6-year-uld children. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:737–746. doi: 10.1016/j.jaac.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovelman I., Gaab N., Christodoulou J.A., Triantafyllou C., Gabrieli J.D.E., Fischl B. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11years of age. Neuroimage. 2010;53:85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries J., Castellanos E.X., Lin H., Zidjdenbos A., Paurs T., Evans A.C., Rapaport J.L., Giedd J.N. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;10:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R. Social buffering of stress in development: a career perspective. Perspect. Psychol. Sci. 2017;12:355–373. doi: 10.1177/1745691616680612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Siemer M., Gotlib I.H. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol. Psychiatr. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavior problems after early life stress: contributions of the Hippocampus and amygdala. Biol. Psychiatr. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., McKlveen J.M., Ghoshal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp. Physiol. 2011;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.H., McCutcheon S.M. Life events checklist. Stress Anxiety. 1980;7:111–125. [Google Scholar]
- Karlsgodt K.H., Shirinyan D., Van Erp T.G.M., Cohen M.S., Cannon T.D. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–1231. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B., Eberly L.E., Westlund Schreiner M., Kurkiewicz P., Houri A., Schlesinger A., Thomas K.M., Mueller B.A., Lim K.O., Cullen K.R. Multilevel assessment of the neurobiological threat system in depressed adolescents: interplay between the limbic system and hypothalamic-pituitary-adrenal axis. Dev. Psychopathol. 2014;26:1321–1335. doi: 10.1017/S0954579414001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss K.J., Mliner S.B., Donzella B., Gunnar M.R. Early adversity, hypocortisolism, and behavior problems at school entry: a study of internationally adopted children. Psychoneuroendocrinology. 2016;66:31–38. doi: 10.1016/j.psyneuen.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryski K.R., Smith H.J., Sheikh H.I., Singh S.M., Hayden E.P. HPA axis reactivity in early childhood: associations with symptoms and moderation by sex. Psychoneuroendocrinology. 2013;38:2327–2336. doi: 10.1016/j.psyneuen.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Kryski K.R., Smith H.J., Sheikh H.I., Singh S.M., Hayden E.P. Assessing stress reactivity indexed via salivary cortisol in preschool-aged children. Psychoneuroendocrinology. 2011;36:1127–1136. doi: 10.1016/j.psyneuen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Schommer N.C., Hellhammer D.H., Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lanteaume L., Khalfa S., Régis J., Marquis P., Chauvel P., Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebr. Cortex. 2007;17:1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Heffelfinger A., Koenig-McNaught A.L., Brown K., Spitznagel E. The preschool feelings checklist: a brief and sensitive screening measure for depression in young children. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:708–717. doi: 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Heffelfinger A., Mrakotsky C., Brown K., Hessler M., Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and No-disorder comparison groups. Arch. Gen. Psychiatr. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Heffelfinger A., Mrakotsky C., Hildebrant T. 1999. Preschool Feelings Checklist. St. Louis, MO. [Google Scholar]
- Magarinos A.M., McEwen B.S. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-L. [DOI] [PubMed] [Google Scholar]
- Magariños A.M., Orchinik M., McEwen B.S. Morphological changes in the hippocampal CA3 region induced by non- invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/S0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Malanchini M., Engelhardt L.E., Raffington L.A., Sabhlok A., Grotzinger A.D., Briley D.A., Madole J.W., Freis S.M., Patterson M.W., Harden K.P., Tucker-Drob E.M. Weak and uneven associations of home, neighborhood, and school environments with stress hormone output across multiple timescales. Mol. Psychiatr. 2020 doi: 10.1038/s41380-020-0747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Merz E.C., Desai P.M., Maskus E.A., Melvin S.A., Rehman R., Torres S.D., Meyer J., He X., Noble K.G. Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biol. Psychiatr. 2019;86:921–929. doi: 10.1016/j.biopsych.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Nugent A.C., Drevets W.C., Dickstein D.S., Leibenluft E., Ernst M., Charney D., Pine D.S. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol. Psychiatr. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mitra R., Sapolsky R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy E., Hernández-Torres E., Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J. Chem. Neuroanat. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Mora F., Segovia G., Del Arco A., De Blas M., Garrido P. Stress, neurotransmitters, corticosterone and body-brain integration. Brain Res. 2012;1476:71–85. doi: 10.1016/j.brainres.2011.12.049. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Petty C.M., Xu Y., Pannu Hayes J., Wagner H.R., Lewis D.V., LaBar K.S., Styner M., McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C., Botteron K.N., Harms M.P., Barch D.M. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Krey L.C., McEwen B.S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schoemaker D., Buss C., Head K., Sandman C.A., Davis E.P., Chakravarty M.M., Gauthier S., Pruessner J.C. Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage. 2016;129:1–14. doi: 10.1016/j.neuroimage.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer L.J., Ziegler T.E., Pollak S.D. Social vocalizations can release oxytocin in humans. Proc. R. Soc. B Biol. Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Send T.S., Bardtke S., Gilles M., Wolf I.A.C., Sütterlin M.W., Kirschbaum C., Laucht M., Witt S.H., Rietschel M., Streit F., Deuschle M. Stress reactivity in preschool-aged children: evaluation of a social stress paradigm and investigation of the impact of prenatal maternal stress. Psychoneuroendocrinology. 2019;101:223–231. doi: 10.1016/j.psyneuen.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Sousa N., Madeira M.D., Paula-Barbosa M.M. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794:199–210. doi: 10.1016/S0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; Stuttgart: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [DOI] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M.C., Eigsti I.M., Thomas K.M., Freed P.J., Booma E.S., Gunnar M.R., Altemus M., Aronson J., Casey B.J. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem Michelle, Korom M., Flannery J., Choy T., Caldera C., Humphreys K.L., Gabard-Durnam L., Goff B., Gee D.G., Telzer E.H., Shapiro M., Louie J.Y., Fareri D.S., Bolger N., Tottenham N. Longitudinal changes in amygdala, hippocampus, and cortisol development following early caregiving adversity. Psyarxiv. 2020 doi: 10.1016/j.dcn.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Shankaranarayana Rao B.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/jneurosci.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.