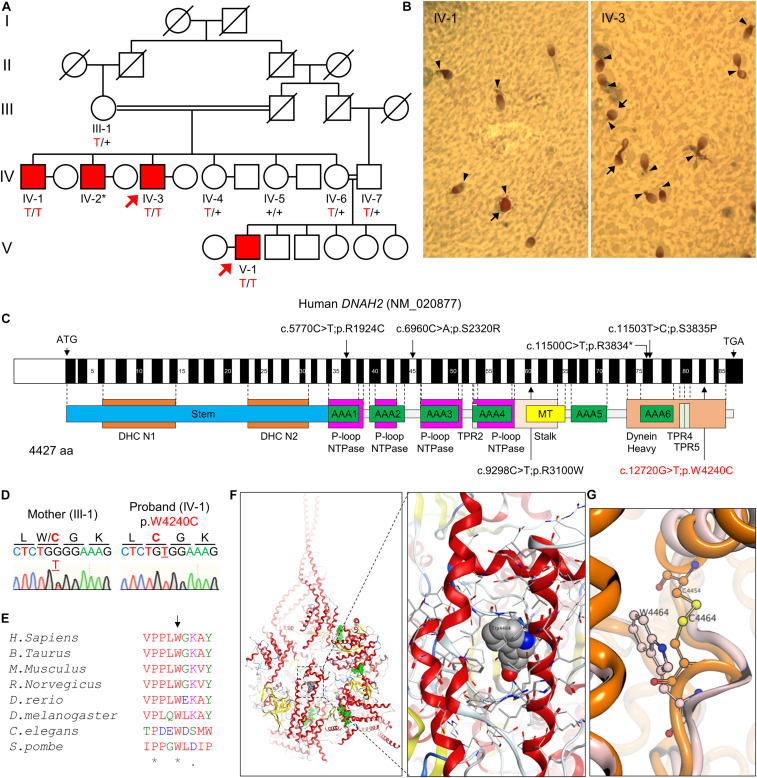
FIGURE 1.
A novel bi-allelic DNAH2 variant identified from a consanguineous family with oligoasthenozoospermia. (A) Pedigree of a consanguineous family with four infertile males (IV-1, IV-2, IV-3, and V-1). WES was performed with IV-3 and V-1 (arrows). Sanger sequencing verified segregation of the variant (red) in the infertile males. A plus (+) indicates a wild-type allele and an asterisk (*) denotes a sample not available for this study. (B) PAP-stained semen from patients IV-1 and IV-3. Multiple morphological defects including near absence of a tail or a short tail (arrowheads), and spherical heads (arrows) are prominent from both patients. (C) Mapping of the DNAH2 variant. Mutation of G to T in exon 82 of DNAH2 cDNA (c.12720G > T; NCBI RefSeq identifier NM_020877) results in a tryptophan-to-cysteine (p.W4240C) substitution in the dynein heavy chain domain. Previously reported pathogenic variants (Li et al., 2019) are also marked (black). (D) Chromatograms of the DNAH2 non-synonymous variant in an infertile sibling (IV-1) and his mother (III-1). The variant is underlined, and the resulting amino acid substitution is marked in red. (E) A sequence alignment of the DNAH2 protein across multiple species. The tryptophan at position 4240 (arrow) is conserved in multiple organisms. An asterisk (*) indicates positions fully conserved and a period (.) indicates positions with weakly similar amino acids. (F,G) Structural modeling of the DNAH2 mutation from this study. (F) Ribbon structure of human DNAH1 (PDB ID: 5NUG), a homolog of human DNAH2. ATP-binding sites are colored in green (left). The enlarged area is the region corresponding to that of W4240 in DNAH2 (right). DNAH1 W4464, which is homologous to W4240, is represented in a space-filling model. (G) Ribbon diagram shows mutation of tryptophan at position 4464 (W4464, orange) to cysteine (W4464C, pink) is predicted to form disulfide bond with nearby C4454 and distort protein backbone structure.