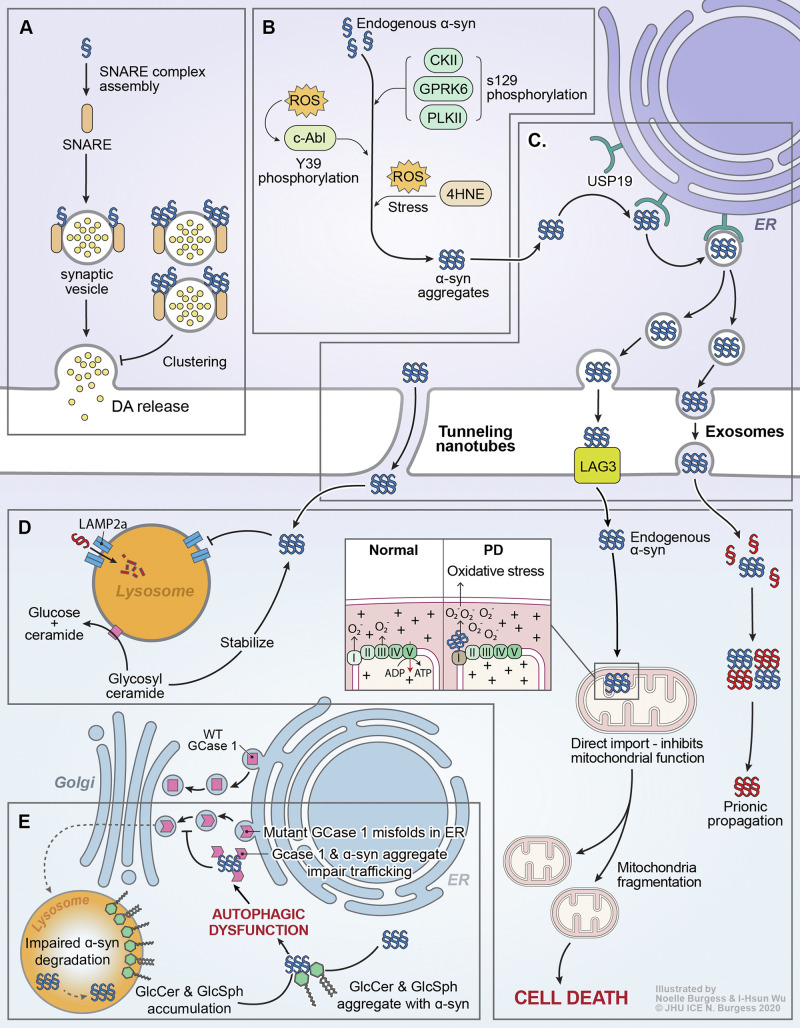
Figure 1.
α-Syn and GBA1 signaling in PD. (A) Monomeric α-syn acts as a chaperone for SNARE proteins, promoting synaptic transmission/DA release. Certain aggregated species of α-syn can reduce DA release by inhibiting synaptic vesicle clustering. (B) Progressive accumulation of α-syn aggregates is a fundamental characteristic of PD progression. Numerous posttranslational modifications have been reported to modulate α-syn aggregation, including pS129 (unclear effect) via multiple kinases, pY39 (pro-aggregation) by c-Abl, and 4-hydroxy-2-nonenal (4HNE) and other modifications mediated by oxidative stress. (C) Aggregated α-syn is incorporated into the ER-transport machinery by USP19 and can propagate from cell to cell via tunneling nanotubes or exosomes, or by direct LAG3-mediated uptake into efferent neurons. (D) Endocytosed α-syn fibrils can seed the templated aggregation of endogenous α-syn and drive prion-like propagation. α-Syn aggregates can also be directly imported into mitochondria, eliciting mitochondrial fragmentation and death. The function of lysosomes, where GCase1 localizes to and metabolizes glycolipids, is also impaired by the presence of α-syn aggregates. (E) GBA1-associated PD is thought to be closely linked to dysregulation of α-syn proteostasis. Likewise, sporadic PD is thought to drive impairments in GCase1 activity. In patients carrying mutant GBA1 alleles, coding mutations in the GCase1 protein may lead to misfolding in the ER, leading to direct coaggregation with α-syn or indirectly causing α-syn aggregation by impairing autophagy. In sporadic PD, loss of GCase1 function may be driven by coaggregation with α-syn, which then serves as a positive feedback loop to accelerate further α-syn aggregation. In addition, accumulation of GCase1 substrates such as GlcCer and glucosylsphingosine (GlcSph) is sufficient to trigger α-syn aggregation.