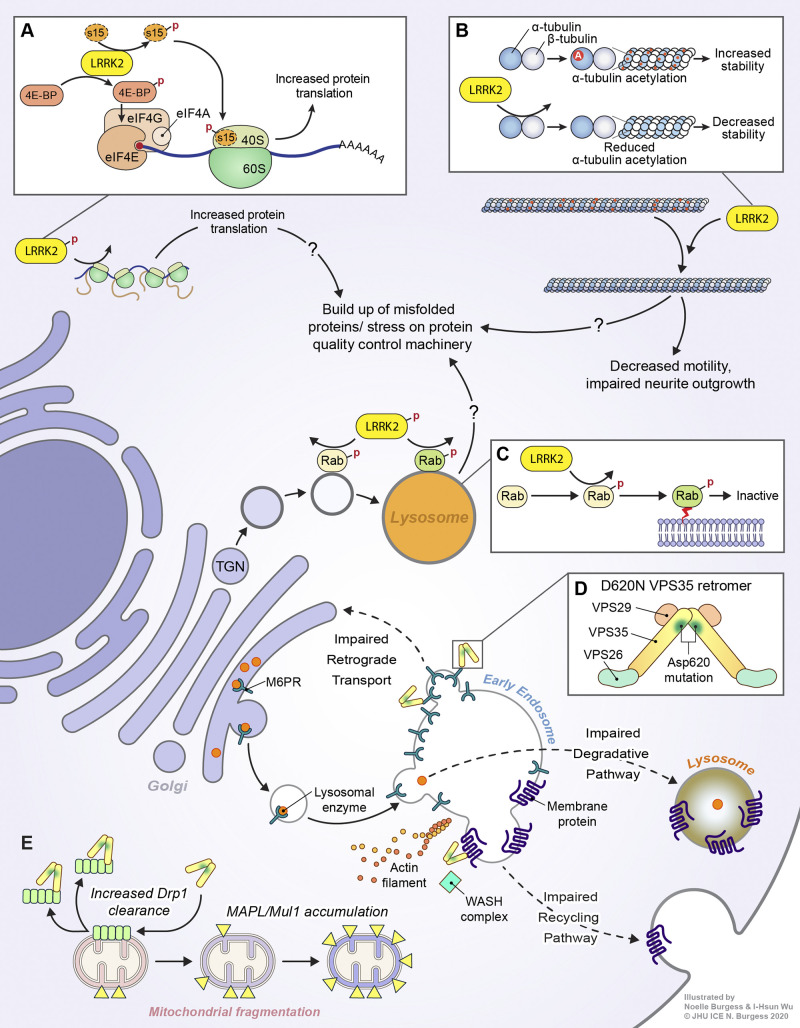
Figure 2.
LRRK2 and VPS35 signaling in PD. (A) LRRK2 may facilitate misfolded protein buildup via increasing global protein translation. It does so by phosphorylating (depicted by a red P) 4E-BP and the 40S ribosomal subunit S15. (B) LRRK2 can associate with β-tubulin via its Roc domain, physically impeding α-tubulin acetylation (depicted by a red A), which may lead to decreased microtubule stability and impaired neurite outgrowth. (C) LRRK2 can phosphorylate multiple RAB proteins within their switch II domains, inactivating them and promoting their membrane targeting. Increased LRRK2 activity may compromise vesicular sorting machinery in neurons, leading to the accumulation of misfolded proteins. (D) The VPS 35 retromer forms a dimer of trimers comprising VPS26/29/35, with the D620N mutation in VPS35 disrupting an acidic residue present at the region critical for retromer complex dimerization, potentially impairing proper assembly of the retromer dimer of trimers. The D620N mutation has been associated with impaired retrograde transport of Golgi-endosome cargo receptors, leading to a deficiency of receptors at the Golgi and impairing forward transport of lysosomal enzymes. D620N VPS35 also exhibits deficiencies in endosome-plasma membrane recycling of surface membrane proteins due to impaired association with the WASH complex. (E) VPS35 is also involved in regulation of mitochondrial dynamics. The D620N mutation can lead to mitochondrial fragmentation through MAPL/Mul1 accumulation or increased Drp1 clearance.