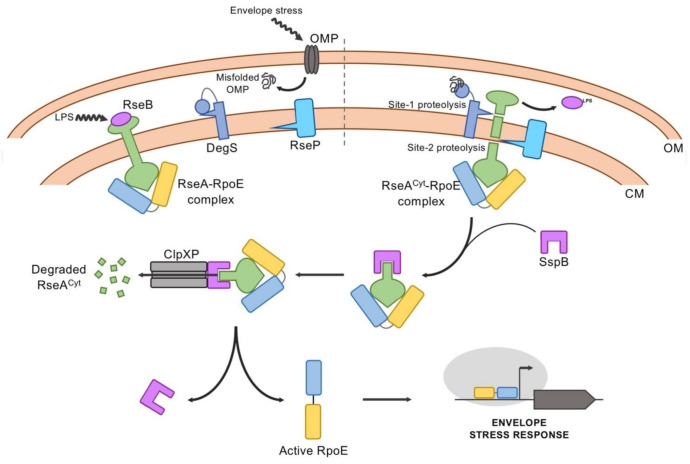
Figure 5.
Regulatory model of the RpoE-mediated envelope stress response. In the absence of stress, RpoE remains sequestered by the membrane-anchored anti-σ factor RseA, which binds the RseB protein in its periplasmic domain. Additionally, the PDZ domain in the protease DegS blocks its own active site. As a first step in the stress response, DegS is able to interact with the C-terminal domain of misfolded OMPs, and off-pathway LPS accumulated in the periplasm relieves RseA from RseB binding. Then, two subsequent proteolytic cleavage events in sites 1 and 2 by proteases DegS and RseP, respectively, release the cytosolic domain of RseA (RseACyt) bound to RpoE to the cytosol. Here, the complex is bound by the adaptor protein SspB that leads it to the major protease ClpXP. This protease degrades the remaining domain of RseA, thus releasing RpoE to drive the expression of its target genes.