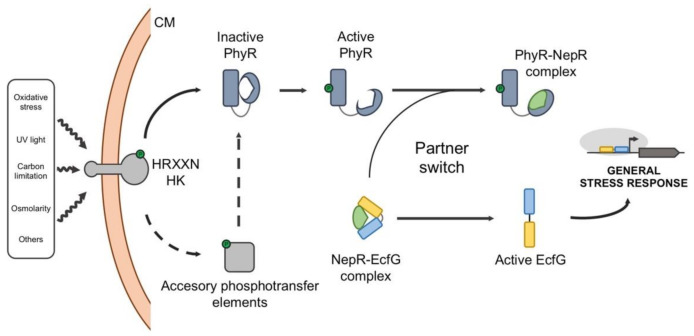
Figure 6.
Regulatory model of the general stress response in Alphaproteobacteria. In the absence of stress, EcfG forms a complex with the anti-σ factor NepR, preventing the activation of the GSR regulon. When some kind of stress appears in the environment, it is sensed by a HRXXN histidine kinase (HK), which autophosphorylates at a His residue and transfers the phosphoryl group to an Asp residue in the response regulator PhyR. Alternatively, the HK may transfer the phosphoryl group to other accessory phosphotransfer elements, such as bifunctional kinases/phosphatases or phosphotransferases, which help to integrate signals from different origins and eventually phosphorylate PhyR in a fine-tuned manner. Upon phosphorylation, PhyR shows a σ-like domain that promotes the partner switch by sequestering NepR. This releases EcfG from inhibition, which is now available to drive the transcription of the GSR regulon.