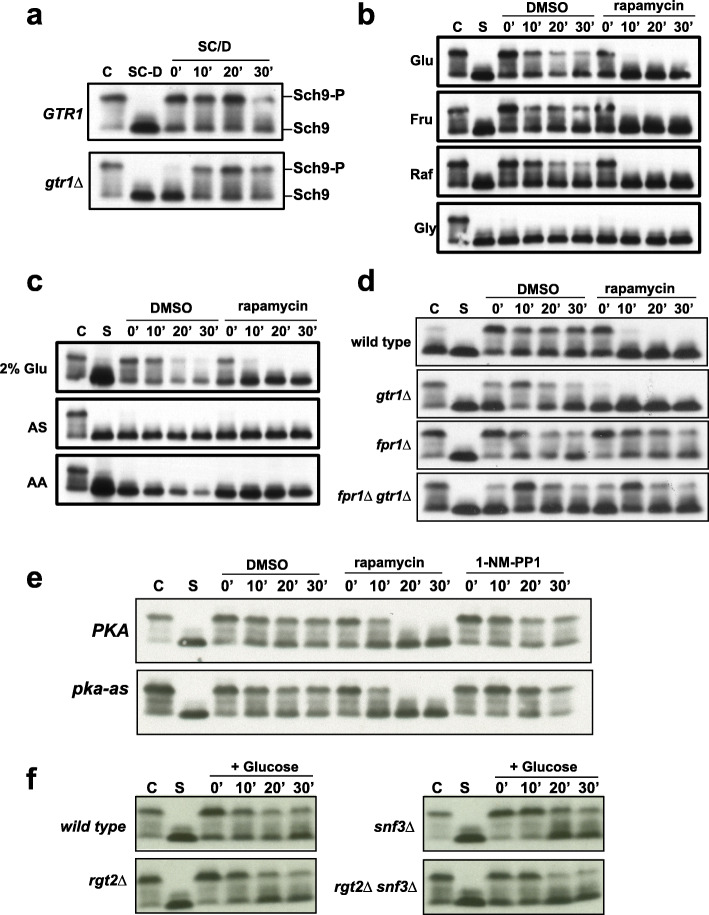
Fig. 2.
Presence of glucose in the medium is necessary and sufficient for TORC1 activation. a Log-phase wild type and gtr1Δ cells (C) grown in synthetic medium with 2% glucose (SC/D) were transferred into synthetic medium lacking glucose (SC-D) and incubated for 1 h. Glucose-starved cells (SC-D) were then transferred back into SC medium (SC/D). Aliquots of the yeast cultures were taken after 0’, 10’, 20’, and 30’ and used for preparing protein extracts. Phosphorylation of Sch9 was monitored by Western blotting. b Wild type cells in logarithmic phase (C) were subjected to complete nutrient starvation by incubating them in 0.3 M sorbitol for 1 h. Starved cells (S) were then transferred to a solution containing either 110 mM glucose or 110 mM fructose, or 110 mM raffinose or 110 mM glycerol in the presence and absence of rapamycin (2 μM). Aliquots of the cultures were taken after 0’, 10’, 20’, and 30’ and used for preparing protein extracts. Phosphorylation of Sch9 was monitored by Western blotting. c Wild type cells in logarithmic phase (C) were subjected to complete nutrient starvation by incubating them in 0.3 M sorbitol for 1 h. Starved cells (S) were then transferred to a solution containing either 110 mM glucose or ammonium sulfate or amino acid mixture in the presence and absence of rapamycin (2 μM). Aliquots of the cultures were taken after 0’, 10’, 20’, and 30’ and used for preparing protein extracts. Phosphorylation of Sch9 was monitored by Western blotting. d Wild type, gtr1Δ, fpr1Δ, and fpr1Δ gtr1Δ cells in log phase were subjected to complete nutrient starvation by incubating them in 0.3 M sorbitol for 1 h. They were then transferred to a 2% glucose solution in the presence and absence of rapamycin (2 μM). Aliquots of the cultures were taken after 0’, 10’, 20’, and 30’ and used for preparing protein extracts. Phosphorylation of Sch9 was monitored by Western blotting. e Wild type and pka-as cells subjected to complete nutrient starvation were transferred to 2% glucose solution in the presence of either DMSO or rapamycin (2 μM) or 1-NM-PP1 (1.5 μM). Aliquots of the cultures were taken after 0’, 10’, 20’, and 30’ and used for preparing protein extracts. Phosphorylation of Sch9 was monitored by Western blotting. f Wild type, rgt2Δ, snf3 Δ, and rgt2Δ snf3Δ cells subjected to complete starvation were transferred to 2% glucose solution. Aliquots of the cultures were taken after 0’, 10’, 20’, and 30’ and used for preparing protein extracts. Phosphorylation of Sch9 was monitored by Western blotting