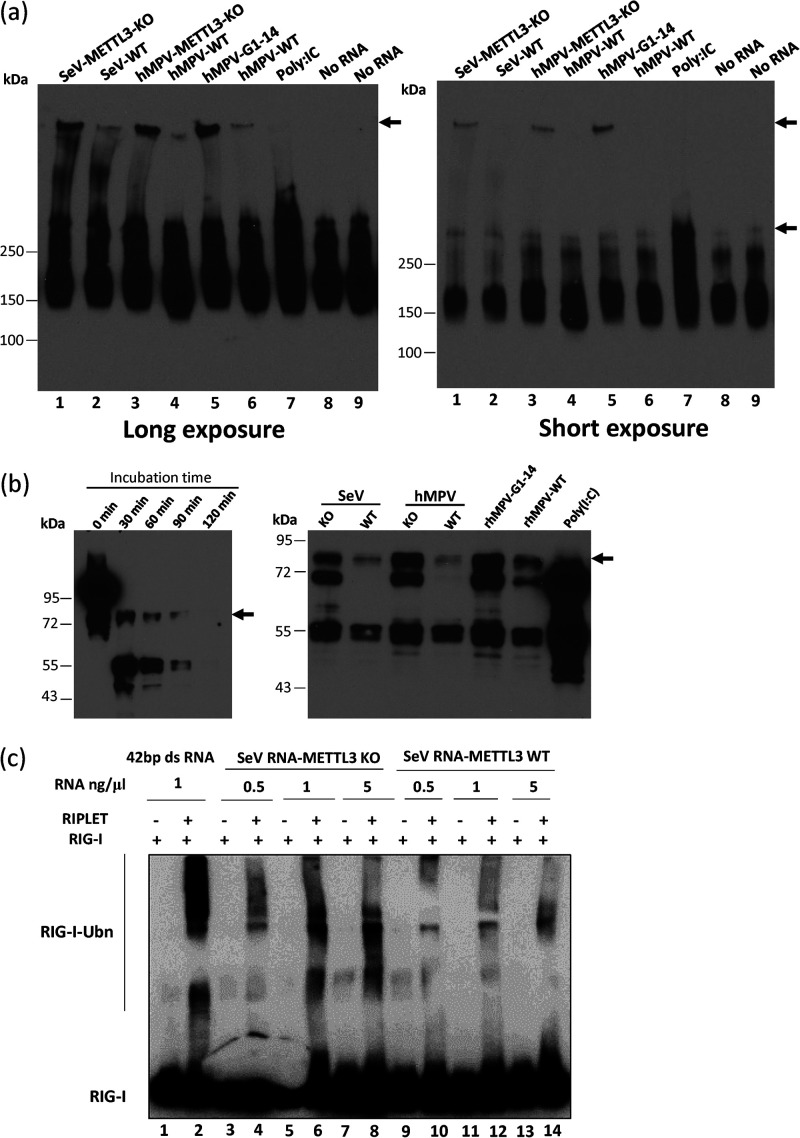
FIG 8.
m6A-deficient virion RNA facilitates RIG-I:RNA conformation change and increases the ubiquitination of RIG-I. (a) Analysis of RIG-I:RNA complex in native PAGE gel. Purified RIG-I was incubated with poly(I·C) or 107 copies of virion RNA in MOPS buffer in the presence of RNase inhibitor and AMP-PNP (2 mM). The reaction mixtures were incubated at 37°C for 30 min to enable RIG-I:RNA complex formation. Ten microliters of the mixture was mixed with an equal volume of native PAGE buffer, and RIG-I:RNA complex was separated in native PAGE gel, followed by Western blotting with anti-RIG-I helicase antibody to detect the RIG-I:RNA complex. Gels with short and long exposures are shown. The Western blots shown are the representatives of three independent experiments. (b) Analysis of RIG-I:RNA conformation by limited trypsin digestion in denaturing SDS-PAGE. The RIG-I:RNA complex was formed as described for panel a. Limited trypsin digestion of RIG-I protein in the absence of RNA ligand for 0 to 2 h (left panel) or in the presence of poly(I·C) or viral RNA (right panel) for 2 h is shown. The Western blots shown are the representatives of three independent experiments. (c) In vitro ubiquitination analysis of RIG-I. A 1.0 μM concentration of purified RIG-I was incubated with 1 ng/μg of 42-bp dsRNA and different doses of SeV virion RNA from METTL3 KO or WT U2OS cells. Ubiquitination of RIG-I was analyzed by anti-RIG-I blotting. The blots shown are the representatives of three independent experiments.