Abstract
Background
Breakdown of the developmentally immature epidermal barrier may permit entry for micro‐organisms leading to invasive infection in preterm infants. Topical emollients may improve skin integrity and barrier function and thereby prevent invasive infection, a major cause of mortality and morbidity in preterm infants.
Objectives
To assess the effect of topical application of emollients (ointments, creams, or oils) on the risk of invasive infection and mortality in preterm infants.
Search methods
We searched CENTRAL via Cochrane Register of Studies (CRS) Web and MEDLINE via Ovid (updated 08 January 2021) and the reference lists of retrieved articles.
Selection criteria
Randomised or quasi‐randomised controlled trials that assessed the effect of prophylactic application of topical emollient on the risk of invasive infection, mortality, other morbidity, and growth and development in preterm infants.
Data collection and analysis
We used the standard methods of Cochrane Neonatal. Two review authors separately evaluated trial quality, extracted data, and synthesised effect estimates using risk ratio (RR), risk difference (RD), and mean difference. We used the GRADE approach to assess the certainty of evidence for effects on mortality and invasive infection.
Main results
We included 22 trials with a total of 5578 infant participants. The main potential sources of bias were lack of clarity on the methods used to generate random sequences and conceal allocation in half of the trials, and lack of masking of parents, caregivers, clinicians, and investigators in all of the trials.
Eight trials (2086 infants) examined the effect of topical ointments or creams. Most participants were very preterm infants cared for in healthcare facilities in high‐income countries. Meta‐analyses suggested that topical ointments or creams may have little or no effect on invasive infection (RR 1.13, 95% confidence interval (CI) 0.97 to 1.31; low certainty evidence) or mortality (RR 0.94, 95% CI 0.82 to 1.08; low certainty evidence).
Fifteen trials (3492 infants) assessed the effect of topical plant or vegetable oils. Most of these trials were undertaken in low‐ or middle‐income countries and were based in healthcare facilities. One large (2249 infants) community‐based trial occurred in a rural field practice in India. Meta‐analyses suggested that topical oils may reduce invasive infection (RR 0.71, 95% CI 0.52 to 0.96; I² = 52%; low certainty evidence) but have little or no effect on mortality (RR 0.94, 95% CI 0.82 to 1.08, I² = 3%; low certainty evidence).
One trial (316 infants) that compared petroleum‐based ointment versus sunflower seed oil in very preterm infants in Bangladesh showed little or no effect on invasive infection (RR 0.91, 95% CI 0.57 to 1.46; low certainty evidence), but suggested that ointment may lower mortality slightly (RR 0.82, 95% CI 0.68 to 0.98; RD ‐0.12, 95% CI ‐0.23 to ‐0.01; number needed to treat for an additional beneficial outcome 8, 95% CI 4 to 100; low certainty evidence). One trial (64 infants) that assessed the effect of coconut oil versus mineral oil in preterm infants with birth weight 1500 g to 2000 g in India reported no episodes of invasive infection or death in either group (very low certainty evidence).
Authors' conclusions
The level of certainty about the effects of emollient therapy on invasive infection or death in preterm infants is low. Since these interventions are mostly inexpensive, readily accessible, and generally acceptable, further good‐quality randomised controlled trials in healthcare facilities, and in community settings in low‐ or middle‐income countries, may be justified.
Keywords: Humans; Infant, Newborn; Administration, Topical; Bacterial Infections; Bacterial Infections/mortality; Bacterial Infections/prevention & control; Bias; Cross Infection; Cross Infection/mortality; Cross Infection/prevention & control; Dermatitis; Dermatitis/prevention & control; Emollients; Emollients/therapeutic use; Infant, Extremely Premature; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/prevention & control; Mycoses; Mycoses/mortality; Mycoses/prevention & control; Ointments; Ointments/therapeutic use; Randomized Controlled Trials as Topic; Skin Care
Plain language summary
Topical emollient for preventing infection in preterm infants
Background
Preterm infants (born before 37 weeks' gestation) are susceptible to bloodstream and other serious infections partly because their immature skin is not a fully effective barrier to micro‐organisms. Applying emollient (ointment, cream, or oil) may protect against skin breakdown and thereby prevent micro‐organisms from spreading into the bloodstream and causing serious infection.
Study characteristics
Our search (updated January 2021) identified 22 eligible trials. In total, 5578 infants participated. Eight trials (2086 infants) examined the effect of topical ointments or creams in very preterm infants (born more than eight weeks early) cared for in hospitals, mostly in high‐income countries. Fourteen trials (3492 infants) assessed the effect of sunflower and other vegetable oils, mostly in low‐ or middle‐income countries in south Asia. All but one of these trials was conducted in hospitals. One large trial in India (2249 infants) was based in the community.
Key results
Regular application of ointments or creams to the skin of very preterm infants may have little or no effect on serious infection or death. Application of sunflower and other vegetable oils may reduce invasive infection but have little or no effect on mortality.
Certainty of evidence
These analyses provide low certainty evidence about the effects of emollient therapy on serious infection or death in preterm infants. Since these interventions are mostly inexpensive, readily accessible, and generally acceptable, further good‐quality randomised controlled trials in healthcare facilities, and in community‐settings in low‐ or middle‐income countries, may be justified.
Summary of findings
Summary of findings 1. Topical ointment or cream versus routine skin care for preventing infection in preterm infants.
| Topical ointment or cream versus routine skin care for preventing infection in preterm infants | ||||||
|
Patient or population: preterm (< 37 weeks') and low birth weight (< 2500 g) infants
Settings: high‐income countries, and low‐ and middle‐income countries
Intervention: topical ointment or cream ¶ Comparison: routine skin care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Routine skin care | Topical ointment or cream | |||||
|
Invasive infection until hospital discharge |
228 per 1000 | 257 per 1000 (221 to 298) | RR 1.13 (0.97 to 1.31) | 2086 (8) | ⊕⊕⊝⊝ lowa,b | Six trials were conducted in high‐income countries, one in a middle‐income country (Turkey), and one in a low‐income country (Bangladesh). No evidence of subgroup difference. |
| Mortality until hospital discharge (or latest reported) | 203 per 1000 | 176 per 1000 (152 to 209) | RR 0.87 (0.75 to 1.03) | 2067 (7) | ⊕⊕⊝⊝ lowa,c | Five trials were conducted in high‐income countries, one in a middle‐income country (Turkey) and one in a low‐income country (Bangladesh). No evidence of subgroup difference. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
¶ Most trials used proprietary emollient, most commonly Aquaphor (a water‐free petroleum‐based ointment) and Bepanthen (a water‐containing lanolin and petroleum‐based ointment). aDowngraded one level due to serious risk of bias (unclear random sequence generation in many trials; caregivers and investigators not masked in any trials). In one trial (Darmstadt 2005), there was a disruption in the method of the randomisation process, which may have contributed to an unequal distribution of infants between groups. bDowngraded one level due to imprecision. 95% CI (0.97 to 1.31) consistent with no effect or substantial harm. cDowngraded one level due to imprecision. 95% CI (0.75 to 1.03) consistent with no effect or substantial benefit.
Summary of findings 2. Topical oil versus routine skin care for preterm infants.
| Topical oil versus routine skin care for preventing infection in preterm infants | ||||||
|
Patient or population: preterm (< 37 weeks) and low birth weight (< 2500 g) infants
Settings: high‐income countries, and low‐ and middle‐income countries
Intervention: topical oil ¶ Comparison: routine skin care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Routine skin care | Topical oil | |||||
| Invasive infection until hospital discharge | 55 per 1000 | 39 per 1000 (29 to 53) | RR 0.71 (0.52 to 0.96) | 3256 (9) | ⊕⊕⊝⊝ lowa,b | Eight trials were conducted in low‐ or middle‐income countries, and one in a high income country (Germany). No evidence of subgroup difference. |
| Mortality until hospital discharge (or latest reported) | 254 per 1000 | 239 per 1000 (208 to 274) | RR 0.94 (0.82 to 1.08) | 1119 (11) | ⊕⊕⊝⊝ lowa,c | Seven trials were conducted in low‐ or middle‐income countries, and two in a high income country (France, Germany). No evidence of subgroup difference. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
¶ Sunflower, sunflower seed, other vegetable oils. aDowngraded one level due to serious risk of bias (unclear random sequence generation or allocation concealment; caregivers and investigators not masked in any trials). bDowngraded one level due to inconsistency. There was evidence of unexplained moderate heterogeneity in this meta‐analysis (I² = 52%). cDowngraded one level due to imprecision. 95% CI (0.82 to 1.08) consistent with potentially important benefit or harm.
Summary of findings 3. Topical ointment or cream versus oil for preterm infants.
| Topical ointment or cream versus oil for preventing infection in preterm infants | ||||||
|
Patient or population: preterm (< 37 weeks) and low birth weight (< 2500 g) infants
Settings: high‐income countries, and low‐ and middle‐income countries
Intervention: topical ointment or cream Comparison: topical oil | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Routine skin care | Topical oil | |||||
| Invasive infection until hospital discharge | 189 per 1000 | 172 per 1000 (108 to 275) | RR 0.91 (0.57 to 1.46) | 316 (1) | ⊕⊕⊝⊝ lowa,b | |
| Mortality until hospital discharge (or latest reported) | 660 per 1000 | 542 per 1000 (449 to 647) | RR 0.82 (0.68 to 0.98) | 316 (1) | ⊕⊕⊝⊝ lowa,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
¶ Sunflower, sunflower seed, other vegetable oils. aDowngraded one level due to serious risk of bias (unclear random sequence generation or allocation concealment; caregivers and investigators not masked in any trials). bDowngraded one level due to imprecision. 95% CI (0.57 to 1.46) consistent with potentially important benefit or harm. cDowngraded one level due to imprecision. 95% CI (0.68 to 0.98) consistent with potentially important benefit or minimal effect.
Summary of findings 4. Topical oil versus another oil for preterm infants.
| Topical oil versus another oil for preventing infection in preterm infants | ||||||
|
Patient or population: preterm (< 37 weeks) and low birth weight (< 2500 g) infants
Settings: high‐income countries, and low‐ and middle‐income countries
Intervention: topical oil Comparison: another topical oil | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Routine skin care | Topical oil | |||||
| Invasive infection until hospital discharge | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 64 (1) | ⊕⊝⊝⊝ verylowa,b | No events |
| Mortality until hospital discharge (or latest reported) | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 64 (1) | ⊕⊝⊝⊝ verylowa,b | No events |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
aDowngraded one level due to serious risk of bias (caregivers and investigators not masked). bDowngraded two levels due to serious imprecision. There were no events in either arm.
Background
Description of the condition
Invasive infection is the most common serious complication associated with intensive care for preterm infants. In high‐income countries, invasive infection occurs in about one‐in‐five very preterm (< 32 weeks) infants, reflecting their duration of exposure to invasive procedures (Samanta 2011; Vergnano 2011; Berrington 2012). Coagulase‐negative staphylococci cause about half of all invasive infections (Isaacs 2003). Other pathogens include gram‐negative bacilli (mainly enteric bacilli), Staphylococcus aureus, enterococci, and fungi (predominantly Candida spp.) (Stoll 2002; Isaacs 2004; Gordon 2006; Camacho‐Gonzalez 2013). The epidemiology of invasive infection in preterm infants in low‐ or middle‐income countries may differ from that in high‐income countries (Zaidi 2005; Khan 2017). The incidence is higher but infections are more commonly due to gram‐negative bacilli and are less likely to be directly associated with intensive care or invasive procedures.
Preterm infants with invasive infection have an elevated risk of mortality and a range of important morbidities including necrotising enterocolitis (NEC), retinopathy of prematurity (ROP), and bronchopulmonary dysplasia (BPD) (Adams‐Chapman 2006; Berrington 2012). Mortality and serious morbidity are usually associated with gram‐negative bacilli, Staphylococcus aureus, enterococcal infection, or fungal infection. Coagulase‐negative staphylococcal infection, although common, is associated with a more benign clinical course. However, even 'low grade' coagulase‐negative staphylococcal bloodstream infection may generate inflammatory cascades associated with both acute morbidity and white matter and other brain damage that may adversely affect neurodevelopment (Stoll 2004).
Description of the intervention
Maturation of the epidermis in utero does not occur until about 34 weeks' gestational age. Although skin maturation is accelerated ex utero, the stratum corneum, eccrine glands, and acid mantle of preterm infants remain physically and functionally immature for several weeks after birth (Harpin 1983). Compared with term infants, preterm infants have few dermal elastic fibres and a weak dermal‐epidermal junction prone to disruption. Very preterm infants, furthermore, lack a vernix caseosa ‐ a mixture of proteins, lipids, and water with anti‐inflammatory and antimicrobial properties (Marchini 2002). As well as increasing the rate of transepidermal evaporative heat loss, immaturity of the epidermal barrier predisposes preterm infants to microbial colonisation and infection (Evans 1986; Rutter 1988; Cartlidge 2000; Rutter 2000). The risk of infection is increased further because preterm infants' fragile skin is susceptible to damage through several mechanisms, including thermal, chemical, adhesive, friction and pressure injuries, as well as iatrogenic skin breaks from blood sampling, cannula placement, or extravasation of intravenously‐administered fluids or medicines (Dyer 2013; Ness 2013).
Emollients
Emollients are moisturising treatments applied directly to the skin to protect the stratum corneum, enhance epidermal barrier function and reduce evaporative losses (Pickens 2000). Most proprietary or commercially‐available emollients are creams (oil‐in‐water suspensions) or ointments (oily creams/water‐in‐oil). Some preparations contain antimicrobial or hydrating agents. Natural vegetable or plant oils (for example, mustard, safflower, sesame, coconut, olive, and soybean oils) have emollient properties and in many countries, particularly in south Asia, application of these to the newborn infant's whole body surface is a widespread traditional practice (Darmstadt 2002a; Darmstadt 2002b).
How the intervention might work
As well as providing a physical barrier to skin disruption, emollient oils, creams, or ointments provide lipids that are integrated into the epidermis to further enhance skin barrier function. Topical oils may also be a transcutaneous nutritional source of essential fatty acids for preterm or low birth weight infants (Lee 1993).
Potential adverse effects of emollients
Although emollients may plausibly improve skin barrier function, the process of application, which may include massage, could disrupt skin integrity in preterm infants. Emollients may also reduce the antimicrobial function of the acid mantle which could potentially increase the risk of colonisation and infection. Many emollients contain excipients which have the potential to be absorbed through the immature epidermal barrier resulting in contact sensitivity, epidermal injury, cutaneous haemorrhagic necrosis, and uraemia (Ness 2013).
Another concern is that emollient preparations may become contaminated and colonised with potential pathogens, particularly preparations stored in non‐sealed containers (BNF for Children 2020). An important practical limitation is that emollients may reduce the effectiveness of adhesives needed to secure intravenous catheters or endotracheal tubes.
Why it is important to do this review
Given the potential for topical emollient therapy to improve skin barrier function and prevent infection in preterm infants, we have assessed the available evidence to inform practice and research.
Objectives
To assess the effect of topical application of emollients (ointments, creams, or oils) on the risk of invasive infection and mortality in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials using random or quasi‐random participant allocation. Cluster‐randomised trials where the unit of randomisation was a group of infants (for example, in a neonatal unit) were eligible for inclusion. Cross‐over studies that assessed the use of emollient therapy in the same infant were not eligible for inclusion as this design would not permit a meaningful assessment of the effect of the intervention on the primary outcome for this review.
Types of participants
Preterm infants (< 37 weeks gestation)
Types of interventions
Ointment or cream versus routine skin care
Oil versus routine skin care
Ointment or cream versus oil
One oil (or combination) versus another oil (or combination)
Types of outcome measures
Primary outcomes
Invasive infection diagnosed more than 48 hours after birth as determined by culture from a normally sterile site: cerebrospinal fluid; blood; urine (obtained by sterile urethral catheterisation or suprapubic bladder tap); bone or joint, peritoneum, pleural space, or central venous line tip; or findings on autopsy examination consistent with invasive microbial infection. If sufficient data were available, we planned to examine specific effects on infection with these organisms:
Coagulase‐negative staphylococci
Other bacteria (gram‐negative bacilli, S aureus, enterococci)
Fungi
Secondary outcomes
Death (all cause) before hospital discharge (in facility‐based trials), or at latest assessment in community trials
Growth: weight gain (g/kg/day); linear growth (mm/week); head circumference (mm/week); skinfold thickness (mm/week) during the trial period
Neurodevelopmental outcomes assessed at more than 12 months post‐term (measured using validated assessment tools) and classifications of disability, including auditory and visual disability. A composite outcome of 'severe neurodevelopmental disability' was defined as any one or combination of the following: non‐ambulant cerebral palsy, severe developmental delay, auditory impairment and visual impairment.
Bronchopulmonary dysplasia (BPD) (oxygen supplementation at 36 weeks' postmenstrual age)
Necrotising enterocolitis (NEC) (Bell stage 2 or 3) (Bell 1978)
Retinopathy of prematurity (ROP) requiring treatment (medical or surgical) (ICCROP 2005)
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search in January 2021 including: Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 1, 01 January 2015 to 08 January 2021) in the Cochrane Library and Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (01 January 2015 to 08 January 2021). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (who.int/ictrp/search/en/), and the United States' National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov) via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry (http://www.isrctn.com/) for any unique trials not found through the Cochrane CENTRAL search.
Previous search details are listed in Appendix 2.
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
One review author (JC) screened titles and abstracts of all records identified by the search and coded records as 'order' or 'exclude'. A second review author (WM) assessed all records coded as 'order' and made the final decision about which records were ordered as full‐text articles. Both authors read the full texts and used a checklist to assess each article's eligibility for inclusion on the basis of pre‐specified inclusion and exclusion criteria.
Data extraction and management
Both authors extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached consensus. If data from the trial reports were insufficient, we contacted trialists for further information.
Assessment of risk of bias in included studies
Both authors independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
We resolved any disagreements through discussion or by consulting a third assessor. See Appendix 3 for a description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in the individual trials using Review Manager 5 (Review Manager 2020), and reported risk ratios (RRs) and risk differences (RDs) for dichotomous data, and mean differences (MDs) for continuous data, with respective 95% confidence intervals (CIs). We determined the number needed to treat for one additional beneficial outcome (NNTB) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually‐randomised trials. For cluster‐randomised trials, we undertook analyses at the level of the individual while accounting for inter‐cluster correlations in the data using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Cross‐over studies were not eligible for inclusion.
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or were reported unclearly. If unavailable, we planned to undertake sensitivity analyses to assess the potential impact of missing outcome data.
Assessment of heterogeneity
We examined treatment effects in individual trials and heterogeneity between trial results by inspecting the forest plots if more than one trial was included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high (I² > 50%) heterogeneity, we planned to explore possible causes (differences in study design, participants, interventions, or outcome assessments).
Assessment of reporting biases
We planned to assess funnel plot asymmetry visually and with Harbord's modification of Egger's test in meta‐analyses with data from more than nine trials contributing events (Harbord 2006).
Data synthesis
We used a fixed‐effect model for meta‐analysis (as per Cochrane Neonatal recommendations). When moderate or high heterogeneity existed, we planned to examine the potential causes in subgroup (see below) and sensitivity (by methodological quality) analyses.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup comparisons.
Very preterm (< 32 weeks) infants versus infants born at 32 weeks gestation or later.
Low‐ and middle‐income versus high‐income countries. (For classification, see: datahelpdesk.worldbank.org/knowledgebase/articles/906519#High_income.)
Sensitivity analysis
We planned sensitivity analyses to determine how estimates of effect on invasive infection and mortality were affected by including only studies at low risk of selection bias (adequate randomisation and allocation concealment), detection or performance bias (adequate masking of intervention and measurement), attrition bias (< 20% loss to follow‐up for primary outcome assessment), or reporting bias (selective reporting).
Summary of findings and assessment of the certainty of the evidence
Both authors (independently) used 'Grading of Recommendations, Assessment, Development and Evaluations' (GRADE) methods to assess the certainty of the evidence for effects on all‐cause mortality and invasive infection (Schünemann 2013). We considered evidence from RCTs as high certainty but 'downgraded' one level for serious (or two levels for very serious) limitations based on: design weaknesses (risk of bias), inconsistency across studies, indirectness, imprecision of estimates, and presence of publication bias. This approach results in an assessment of the certainty of a body of evidence as one of four grades:
high certainty: we have a lot of confidence that the true effect is similar to the estimated effect; further research is very unlikely to change our confidence in the estimate of effect;
moderate certainty: we believe that the true effect is probably close to the estimated effect; further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low certainty: the true effect might be markedly different from the estimated effect; further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low certainty: the true effect is probably markedly different from the estimated effect; we are very uncertain about the estimate.
We used GRADEpro GDT software to create ‘Summary of findings’ tables to report the certainty of the evidence.
Results
Description of studies
Results of the search
See Figure 1.
1.
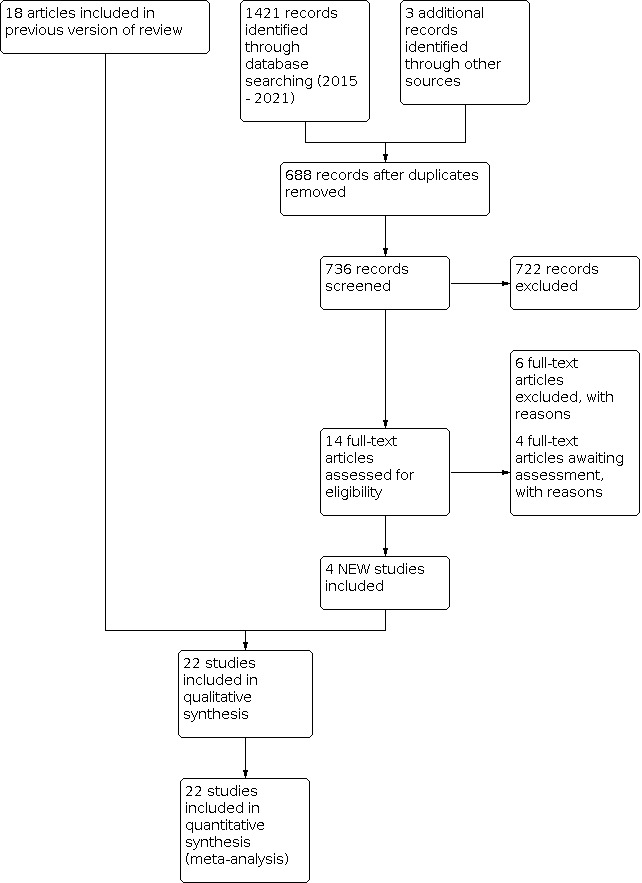
Study flow diagram: 2021 review update
Included studies
We included trial reports from 22 primary publications (Characteristics of included studies). Most reports were of two‐arm trials. Four were of three‐arm trials (Arora 2005; Darmstadt 2005; Sankaranarayanan 2005; Kiechl‐Kohlendorfer 2008).
Participants and setting
The included trials were conducted since the late 1990s in neonatal care centres in high‐income countries (USA, Saudi Arabia, Germany, Austria, Australia, and France), middle‐income countries (Turkey, India, Egypt, Iran, and Brazil), and a low‐income country (Bangladesh).
In total, 5578 infants participated. Most trials were single‐centre and facility‐based. One large (N = 2249) community‐based trial occurred in a "rural field practice" in West Bengal, India between 2014 and 2018 (Konar 2019).
Birth weight or gestational age inclusion criteria:
Lane 1993: 29 to 36 weeks
Nopper 1996: less than 33 weeks
Pabst 1999: 26 to 30 weeks
Soriano 2000: less than 1700 g
Darmstadt 2004: less than 34 weeks
Edwards 2004: less than 31 weeks and 501 g to 1000 g
Arora 2005: less than 1500 g
Darmstadt 2005: less than 33 weeks
Sankaranarayanan 2005: 1500 g to 2000 g
Kiechl‐Kohlendorfer 2008: 25 to 36 weeks
Vaivre‐Douret 2008: 31 to 34 weeks
Farhat 2010: less than 2000 g and less than 37 weeks
Fallah 2013: 1500 g to 1999 g and 33 to 37 weeks
Kumar 2013: less than 1800 g and less than 35 weeks
Alkharfy 2014: less than 1250 g and less than 33 weeks
Kanti 2014: 1500 g to 2500 g
Erdemir 2015: less than 34 weeks
Salam 2015: 26 to 36 weeks and more than 750 g
Jabraeile 2016: 1500 g to 1000 g and 28 to 32 weeks
Kukreja 2018: 1000 g to 2000 g
Strunk 2018; less than 30 weeks
Konar 2019: less than 37 weeks
Interventions
The intervention was generally commenced within a few days after birth and continued until about one to four weeks postnatally, or until hospital discharge. The ointments or oils were massaged between two and six times each day into the whole skin surface (except the face or head) by either the infant's mother or nurse or other caregiver.
Comparison 1
Eight trials compared treatment with emollient ointment (mainly proprietary preparations) versus routine skin care:
Aquaphor(Nopper 1996; Pabst 1999; Edwards 2004; Darmstadt 2005; Erdemir 2015)
Bepanthen(Kiechl‐Kohlendorfer 2008)
Eucerin (Lane 1993)
petroleum jelly (Alkharfy 2014)
olive oil/lanolin cream (Kiechl‐Kohlendorfer 2008)
Comparison 2
Fifteen trials compared treatment with a natural vegetable or plant oil versus routine skin care:
sunflower oil (Darmstadt 2004; Arora 2005; Darmstadt 2005; Farhat 2010; Fallah 2013; Kumar 2013; Kanti 2014; Kukreja 2018)
coconut oil (Sankaranarayanan 2005; Salam 2015; Strunk 2018; Konar 2019)
soybean oil (Soriano 2000)
almond oil or vegetable oil (Vaivre‐Douret 2008)
olive oil (Jabraeile 2016)
Comparison 3
One trial compared ointment or cream versus topical oil:
Aquaphor versus sunflower seed oil (Darmstadt 2005)
Comparison 4
One trial compared one oil with another oil
coconut oil versus mineral oil (Sankaranarayanan 2005)
Outcomes
Most trials reported data on invasive infection and mortality. Several trials of topical oil versus standard care primarily assessed growth parameters but unpublished data on the rate of infection and death were available from the study investigators (Soriano 2000; Arora 2005; Sankaranarayanan 2005; Kumar 2013; Kukreja 2018; Konar 2019). None of the studies assessed any growth or neurodevelopmental outcomes beyond infancy.
Excluded studies
We screened the full texts of 32 articles of studies which did not meet inclusion criteria (see Characteristics of excluded studies). Five potentially eligible studies remain to be assessed when further information is available from the authors or via publication of the full report (Hu 2014; Saeidi 2014; Nangia 2015; Maamouri 2018; Summers 2019).
Risk of bias in included studies
See Figure 2
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
None of the included trials employed quasi‐random allocation methods, but the methods used to generate the random sequence and conceal allocation are not described in about half of the trial reports.
Blinding
The caregivers and investigators were not masked to the intervention in any of the trials.
Incomplete outcome data
Attrition bias does not appear to be an issue in most trials (outcome data reported for > 80% of randomised cohorts).
Selective reporting
Most reports did not provide access to the trial protocol. It is unlikely, however, that reporting bias was an issue in most trials (low risk of bias) where the review's primary and infant‐important outcomes were reported. In some trials, where the aim was to assess surrogate outcomes such as skin condition or hydration, clinical outcome data were generally available from the investigators.
Other potential sources of bias
We did not find evidence of important between‐group differences in reported baseline characteristics in any of the included trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1. Topical ointment or cream versus routine skin care
Invasive infection
Meta‐analysis did not show an effect (RR 1.13, 95% CI 0.97 to 1.31; I² = 36%; 8 trials, 2086 infants). There was no evidence of subgroup differences in trials conducted in low‐ and middle‐income versus high‐income countries (Chi² = 1.97, df = 1 (P = 0.16); Analysis 1.1; Figure 3). Sensitivity meta‐analysis of trials at low risk of selection, attrition, or reporting bias showed a higher risk of infection in the intervention group (RR 1.18, 95% CI 1.01 to 1.38; I² = 12%; RD 0.04, 95% CI 0.00 to 0.08; 5 trials, 1834 infants).
1.1. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 1: Invasive infection (any organism)
3.

Forest plot of comparison: 1 Topical ointment or cream versus routine skin care, outcome: 1.1 Invasive infection (any organism).
Two trials (1210 infants) restricted participation to very preterm infants (Pabst 1999; Edwards 2004). Meta‐analysis showed a higher rate of invasive infection in the intervention group (RR 1.25, 95% CI 1.04 to 1.50; I² = 32%; RD 0.06, 95% CI 0.01 to 0.11; number needed to treat for an additional harmful outcome (NNTH) 17, 95% CI 9 to 100; Analysis 1.2). The other trials did not report subgroup data for very preterm infants.
1.2. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 2: Invasive infection (trials with only very preterm infants participating)
We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 1).
Infection with specific organisms
Six trials (1839 infants) provided data on the type of infecting organism (Lane 1993; Nopper 1996; Pabst 1999; Edwards 2004; Darmstadt 2005; Erdemir 2015). The meta‐analyses showed a higher risk of infection with coagulase‐negative staphylococci but not infection with other bacteria or fungi:
Coagulase‐negative staphylococci: RR 1.30, 95% CI 1.03 to 1.65; I² = 36% (Analysis 1.3)
Other bacteria (gram‐negative bacilli, S. aureus, enterococci): RR 0.84, 95% CI 0.63 to 1.12; I² = 0% (Analysis 1.4)
Fungi: RR 1.27, 95% CI 0.78 to 2.06, I² = 0% (Analysis 1.5)
1.3. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 3: Invasive infection (coagulase negative staphylococci)
1.4. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 4: Invasive infection (other bacteria)
1.5. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 5: Invasive infection (fungi)
Mortality
Meta‐analysis did not show an effect (RR 0.87, 95% CI 0.75 to 1.03; I² = 39%; 6 trials, 2067 infants). There was no evidence of subgroup differences in trials conducted in low‐ and middle‐income versus high‐income countries (Chi² = 0.80, df = 1 (P = 0.37); Analysis 1.6; Figure 4). Sensitivity meta‐analysis of trials at low risk of selection, attrition, or reporting bias did not show an effect (RR 0.87, 95% CI 0.74 to 1.02; I² = 54%; 5 trials, 1834 infants).
1.6. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 6: Mortality
4.

Forest plot of comparison: 1 Topical ointment or cream versus routine skin care, outcome: 1.6 Mortality.
One trial restricted participation to very preterm infants (Edwards 2004). Analysis did not show a difference (RR 0.90, 95% CI 0.65 to 1.23; 1 trial, 1191 infants; Analysis 1.7). The other trials did not report subgroup data for very preterm infants.
1.7. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 7: Mortality (trials with only very preterm infants participating)
We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 1).
Growth
Not reported.
Neurodevelopmental outcomes
Not reported.
BPD
Meta‐analysis did not show a difference (RR 1.00, 95% CI 0.88 to 1.14; I² = 29%; 2 trials, 1009 infants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 8: BPD
NEC
Meta‐analysis did not show a difference (RR 1.25, 95% CI 0.89 to 1.76; I² = 0%; 4 trials, 1472 infants; Analysis 1.9).
1.9. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 9: NEC
ROP
Analysis did not show a difference (RR 0.99, 95% CI 0.77 to 1.28; 1 trial, 952 infants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Topical ointment or cream versus routine skin care, Outcome 10: ROP (severe)
Comparison 2. Topical oil versus routine skin care
Invasive infection
Meta‐analysis showed a lower rate in the intervention group (RR 0.71, 95% CI 0.52 to 0.96; I² = 52%; RD ‐0.02, 95% CI ‐0.03 to 00.0; 9 trials, 3256 infants). There was no evidence of subgroup differences in trials conducted in low‐ and middle‐income versus high‐income countries (Chi² = 0.54, df = 1 (P = 0.46); Analysis 2.1; Figure 5). Sensitivity meta‐analysis of trials at low risk of selection, attrition, or reporting bias did not show an effect (RR 0.91, 95% CI 0.65 to 1.30; I² = 24%; 6 trials, 1914 infants).
2.1. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 1: Invasive infection (any organism)
5.

Forest plot of comparison: 2 Topical oil versus routine skin care, outcome: 2.1 Invasive infection (any organism).
One trial (78 infants) restricted participation to very preterm infants (Strunk 2018). Analysis did not show an effect (RR 0.40, 95% CI 0.08 to 1.93; Analysis 2.1). The other trials did not report subgroup data for very preterm infants.
Darmstadt 2004 stated that the rate of invasive infection was lower in the intervention group but did not report the number of infants in each group who had at least one episode of invasive infection. These data are not available from the principal investigator.
We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (lack of masking) and inconsistency (Table 2).
Infection with specific organisms
Four reports provided data on the type of infecting organism (Darmstadt 2005; Salam 2015; Kukreja 2018; Strunk 2018). None of the participants in three other trials had an episode of invasive infection (Soriano 2000; Sankaranarayanan 2005; Kanti 2014). None of the meta‐analyses (7 trials, 893 infants) showed an effect:
Coagulase‐negative staphylococci: RR 0.22, 95% CI 0.05 to 1.02; I² = 0% (Analysis 2.2)
Other bacteria (gram‐negative bacilli, Staphylococcus aureus, enterococci): RR 0.71, 95% CI 0.48 to 1.04; I² = 0% (Analysis 2.3)
Fungi: typical RR 1.93, 95% CI 0.42 to 8.78; I² = 52% (Analysis 2.4)
2.2. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 2: Invasive infection (coagulase negative staphylococci)
2.3. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 3: Invasive infection (other bacteria)
2.4. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 4: Invasive infection (fungi)
Mortality
Meta‐analysis did not show an effect (RR 0.94, 95% CI 0.82 to 1.08; I² = 3%; 11 trials, 1119 infants). There was no evidence of subgroup differences in trials conducted in low‐ and middle‐income versus high‐income countries (Chi² = 2.11, df = 1 (P = 0.15); Analysis 2.5; Figure 6). Sensitivity meta‐analysis of trials at low risk of selection, attrition, or reporting bias did not show an effect (RR 0.94, 95% CI 0.82 to 1.09; I² = 44%; 5 trials, 621 infants).
2.5. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 5: Mortality
6.

Forest plot of comparison: 2 Topical oil versus routine skin care, outcome: 2.5 Mortality.
One trial (78 infants) restricted participation to very preterm infants (Strunk 2018). Analysis did not show an effect (RR 0.11, 95% CI 0.01 to 1.99; Analysis 2.5). The other trials did not report subgroup data for very preterm infants.
Darmstadt 2004 reported infection‐attributed mortality but not all‐cause mortality data. We contacted the principal investigator but this information is not available for inclusion.
We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 2).
Growth during the trial period
Data were available from seven trials (Soriano 2000; Arora 2005; Sankaranarayanan 2005; Farhat 2010; Fallah 2013; Kumar 2013; Jabraeile 2016). Meta‐analyses showed that infants in the emollient group had higher rates of weight gain (MD 2.93 g/kg/day, 95% CI 2.11 to 3.76; I² = 62%) and linear growth (MD 1.34 mm/week, 95% CI 0.20 to 2.47; I² = 0%), but not head circumference growth (MD 0.66 mm/week, 95% CI ‐0.54 to 0.70; I² = 0%) or triceps skinfold thickness rate of change (MD 0.04 mm/week, 95% CI ‐0.03 to 0.11; I² = 0%; Analysis 2.6).
2.6. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 6: Growth
Two trials reported data on weight change that could not be meta‐analysed:
Strunk 2018: median weight after 21 days of infants in the emollient group was 100 g higher than the controls
Kukreja 2018: average weight loss during the 10 days study period was not different (41 g/kg versus 39 g/kg)
Sensitivity analysis
The moderate heterogeneity (I² = 62%) in the meta‐analysis of effect on weight gain is attributed to one 'outlier' (Jabraeile 2016). Exclusion of this trial removes the heterogeneity, but the effect size is similar (MD 2.62 g/kg/day, 95% CI 1.78 to 3.47; I² = 0%). Participants and interventions were similar to the other trials, and quality assessment scores were similar with the exception of allocation concealment. Investigators are likely to have been prospectively aware of group allocation. The mean weight at baseline differed between the groups (1321 g versus 1145 g; MD 176 g, 95% CI 119 to 233), which may have affected care practices and rate of weight gain over the ensuing 10 days.
Neurodevelopmental outcomes
One trial assessed neurodevelopment beyond infancy (Strunk 2018). Analysis did not show a difference in the prevalence of moderate or severe neurodevelopmental delay at 24 months (corrected age) in cognitive, language, motor or socio‐emotional domains assessed using Bayley Scales of Infant Development (3rd Edition) (Analysis 2.7):
2.7. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 7: Moderate‐severe neurodevelopmental delay
Cognitive: RR 0.25, 95% CI 0.06 to 1.11
Language: RR 0.48, 95% CI 0.21 to 1.11
Motor: RR 0.25, 95% CI 0.06 to 1.11
Socio‐emotional: RR 0.30, 95% CI 0.07 to 1.33
BPD
One trial (72 infants) assessed BPD (Strunk 2018). Analysis did not show a difference: RR 0.93, 95% CI 0.53 to 1.64 (Analysis 2.8).
2.8. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 8: BPD
NEC
One trial (72 infants) assessed NEC (Strunk 2018). Analysis did not show a difference: RR 0.20, 95% CI 0.01 to 4.03 (Analysis 2.9).
2.9. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 9: NEC
ROP
One trial (72 infants) assessed ROP (Strunk 2018). Analysis did not show a difference: RR 1.00, 95% CI 0.27 to 3.69 (Analysis 2.10).
2.10. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 10: ROP (severe)
Comparison 3. Topical ointment or cream versus oil
Invasive infection
One trial (316 infants) reported invasive infection (Darmstadt 2005). Analysis did not show a difference: RR 0.91, 95% CI 0.57 to 1.46 (Analysis 3.1).
3.1. Analysis.

Comparison 3: Topical ointment or cream vs. topical oil., Outcome 1: Invasive infection (any organism)
We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 3).
Infection with specific organisms
(i) Coagulase‐negative staphylococci: no events detected (Analysis 3.2).
3.2. Analysis.

Comparison 3: Topical ointment or cream vs. topical oil., Outcome 2: Invasive infection (coagulase negative staphylococci)
(ii) Other bacteria (gram‐negative bacilli, Staphylococcus aureus, enterococci): RR 0.90, 95% CI 0.53 to 1.50 (Analysis 3.3).
3.3. Analysis.

Comparison 3: Topical ointment or cream vs. topical oil., Outcome 3: Invasive infection (other bacteria)
(iii) Fungi: RR 1.35, 95% CI 0.31 to 5.94 (Analysis 3.4).
3.4. Analysis.

Comparison 3: Topical ointment or cream vs. topical oil., Outcome 4: Invasive infection (fungi)
Mortality
One trial (316 infants) reported mortality (Darmstadt 2005). Analysis showed a reduced risk in the ointment/cream group: RR 0.82, 95% CI 0.68 to 0.98; RD ‐0.12, 95% CI ‐0.23 to ‐0.01; number needed to treat for an additional beneficial outcome (NNTB) 8, 95% CI 4 to 100 (Analysis 3.5).
3.5. Analysis.

Comparison 3: Topical ointment or cream vs. topical oil., Outcome 5: Mortality
We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 3).
Growth outcomes
Not reported.
Neurodevelopmental outcomes
Not reported.
BPD
Not reported.
NEC
Not reported.
ROP
Not reported.
Comparison 4. Topical oil versus another oil
Invasive infection
One trial (64 infants) reported invasive infection (Sankaranarayanan 2005). No events were detected (Analysis 4.1).
4.1. Analysis.

Comparison 4: One topical oil (or combination) vs. another oil (or combination), Outcome 1: Invasive infection
We assessed the certainty of evidence as very low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 4).
Mortality
One trial (64 infants) reported mortality (Sankaranarayanan 2005). No events were detected (Analysis 4.2).
4.2. Analysis.

Comparison 4: One topical oil (or combination) vs. another oil (or combination), Outcome 2: Mortality
We assessed the certainty of evidence as very low using GRADE methods, downgraded for serious study design limitations (lack of masking) and imprecision (Table 4).
Growth during the trial period
One trial (64 infants) assessed growth parameters (Sankaranarayanan 2005). Analysis showed a higher rate of weight gain in the coconut oil group compared to the mineral oil group (MD 2.00 g/kg/day, 95% CI 0.84 to 3.16). Rates of change in length (MD 0.40 mm/week, 95% CI ‐0.29 to 1.09) and head circumference (MD 0.10 mm/week, 95% CI ‐0.17 to 0.37) were not different (Analysis 4.3).
4.3. Analysis.

Comparison 4: One topical oil (or combination) vs. another oil (or combination), Outcome 3: Growth
Neurodevelopmental outcomes
Not reported.
BPD
Not reported.
NEC
Not reported.
ROP
Not reported.
Discussion
Summary of main results
Topical ointment or cream versus routine skin care
We included eight randomised controlled trials in which a total of 2086 preterm infants participated. The trials were undertaken during the past 25 years. Six were conducted in high‐income countries, one in a middle‐income country (Turkey) and one in a low‐income country (Bangladesh). Most trials used proprietary emollient, most commonly Aquaphor (a water‐free petroleum‐based ointment) and Bepanthen (a water‐containing lanolin and petroleum‐based ointment). Most participants were preterm infants born before 32 weeks gestation, and in the largest trial, all participants were extremely low birth weight (N = 1191) (Edwards 2004).
Meta‐analyses did not show differences in the risk of invasive infection, mortality or morbidity (BPD, ROP, NEC) before discharge from hospital. We did not find evidence of a subgroup effect in a pre‐specified analysis of trials set in high‐income versus low‐ or middle‐income countries. The certainty of the evidence for the effect on infection was assessed as low by GRADE methods because of concerns about the risk of selection, performance or detection bias in the included trials, and imprecision of the effect estimates.
Topical oil versus routine skin care
We included 15 randomised controlled trials in which a total of 3492 preterm infants participated. The trials were undertaken within the past 25 years. Nine were conducted in low‐ or middle‐income countries, and two in high‐income countries (Vaivre‐Douret 2008; Kanti 2014). The most commonly used emollients were sunflower and other plant or vegetable oils. All but one of the trials were based in healthcare facilities. One large (2249 infants) community‐based trial occurred in a rural field practice in West Bengal, India (Konar 2019). Most of the trials had some methodological limitations, particularly lack of masking of caregivers and clinicians, and uncertainty about the mechanics of the randomisation procedure.
Meta‐analysis showed a lower rate of invasive infection in the intervention group, but this analysis showed moderate statistical heterogeneity (I² = 52%). A sensitivity meta‐analysis of trials at low risk of bias did not show an effect. Meta‐analyses did not show differences in mortality or other morbidity.
Infants massaged with vegetable oil had a higher rate of weight gain (about 2 g/kg/day) and linear growth (about 0.8 mm/week), though not head growth. These meta‐analysis contained considerable heterogeneity. There are not yet any data at all on long‐term growth and developmental outcomes.
Topical ointment or cream versus topical oil
Only one trial compared ointment (Aquaphor) versus sunflower seed oil (Darmstadt 2005). Analysis suggested a reduction in mortality in the ointment group but no evidence of an effect on the invasive infection rate.
Different oils
One small trial that compared coconut oil versus mineral oil did not detect any episodes of invasive infection or mortality in either group (Sankaranarayanan 2005).
Overall completeness and applicability of evidence
Topical ointment or cream versus routine skin care
These data provide low certainty evidence that topical ointments or creams may have little or no effect on invasive infection or mortality in preterm infants. Analysis of trials set in high‐income countries suggests that routine topical application of ointments might increase the risk of infection. These trials recruited predominantly very preterm infants and most participants in the largest trial were of extremely low birth weight. This finding is consistent with those of a recent Cochrane Review that suggested that use of topical emollients during the first year of life in healthy term infants probably increases the risk of skin infection, perhaps related to suboptimal hand hygiene practices (Kelleher 2021).
The commonest organisms causing bloodstream infection were coagulase‐negative staphylococci. Although some care practices, including infection control measures, feeding policies, and exposure to invasive procedures, may have changed since the larger trials were conducted more than 20 years ago, these findings are likely to remain applicable to the modern context of neonatal intensive care in high‐income countries. A plausible mechanism for the increased risk of infection with coagulase‐negative staphylococci is that application of the ointment causes skin trauma and epidermal micro‐abrasion which permits transcutaneous migration of skin commensals. This is not consistent, however, with the finding in these trials that topical emollient improves skin condition as measured by skin score and evaporative water loss. Another possibility is that contamination may have occurred during the application process and that the ointment provided an environment conducive to the proliferation of bacteria. Analyses did not demonstrate an effect on infection due to other bacteria (gram‐negative bacilli, enterococci, S. aureus) or fungi but these infections occurred infrequently compared with coagulase‐negative staphylococcal infection and the 95% CI for these estimates are broad. Larger trials would be needed to obtain more precise estimates of the specific effect on these less common, though much more virulent, infections.
The finding from one trial that treatment with a topical ointment (Aquaphor), compared to routine skin care or application of vegetable oil, results in a substantial reduction in neonatal mortality should be interpreted and applied cautiously (Darmstadt 2005). Uncertainty exists concerning the method used to randomly allocate participants (discussed below).
Topical oil versus routine skin care
Trial data suggest that treatment with vegetable oils versus standard skin care may reduce the risk of invasive infection in preterm infants. This finding should be interpreted with caution as the 95% CI around the point estimate is broad and the meta‐analysis is moderately heterogeneous. More precise estimates of effect sizes may be obtained when further data from a large ongoing trial are available (Kumar 2020).
The mechanism by which massage with vegetable oils increases the rates of weight and length gain is not clear. Transcutaneously absorbed lipids may be an additional source of calories or essential fatty acids. Reducing evaporative heat loss is another plausible mechanism. Applying topical oils by massage may have a calming effect that reduces energy expenditure or promotes more effective enteral feeding behaviours. Given the high risk of nutritional compromise that exists for preterm infants, particularly in low‐ and middle‐income countries, it may be appropriate to undertake further trials to assess whether topical vegetable oils may have clinically important benefits during this potentially critical phase of growth and growth‐programming (Mullany 2005).
Quality of the evidence
We assessed the evidence for the main outcomes (risk of infection and death) to be low certainty. Many of the trials contained methodological weaknesses; specifically, uncertainty about adequate allocation concealment methods in about half of the trials and lack of masking in all of the trials. Parents, caregivers, clinicians, and investigators were likely to have been aware of the treatment group to which infants had been allocated and this knowledge may have affected some care‐giving practices or investigation strategies, including thresholds for screening for invasive infection, that may have affected the outcomes assessed.
A further concern exists with regard to the random allocation process used in one of the largest of the included trials (Darmstadt 2005). The report states that infants were randomised within "blocks of six with two assignments per block for all three of the groups". This form of block randomisation would be expected to generate roughly equal numbers of participants in each of the three study arms. However, the report indicates unequal distribution of infants. The principal investigator has provided further information (post hoc withdrawal of a fourth arm and change in the sequence generation and allocation methods) that may have contributed to this discrepancy. Given the potential for these post hoc changes to have disrupted the integrity of the randomisation process, it may be most appropriate to interpret and apply the findings of this trial with caution.
Potential biases in the review process
The main concern with the review process is the possibility that the findings are subject to publication and other reporting biases. We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of the major international perinatal conferences to identify trial reports that are not (or not yet) published in full form in academic journals. The meta‐analyses that we performed did not contain sufficient trials to explore symmetry of funnel plots as a means of identifying possible publication or reporting bias.
We have not been able to obtain data from two trials for inclusion in meta‐analyses. Darmstadt 2004 did not report the number of infants in each group who acquired an infection (only the total number of infections which includes multiple infections in individual infants) or all‐cause mortality (only infection‐attributed mortality). These data have not yet been made available by the investigators. One trial has been completed but has yet to report methodological details and numerical data (Hu 2014). Four other potentially eligible trials have not reported clinical outcome data. We have sought these data from the investigators and, when available, we will include them in an updated version of this review.
Agreements and disagreements with other studies or reviews
Our findings are broadly consistent with other systematic reviews of topical emollient for preventing infection in preterm infants (Salam 2013; Pupala 2019). Our review differs from others in some respects:
we included trials that assessed any emollient, but pre‐specified separate comparisons of ointments/creams and oils;
we conducted subgroup analyses to explore differences in effect sizes depending upon whether the trial was set in a low‐ or middle‐income country versus a high‐income country;
we pre‐specified sensitivity analyses to determine how trial methodological quality affected effect size estimates; and
we included a GRADE assessment of the certainty of the evidence at outcomes level to help inform policy, practice, and research.
Authors' conclusions
Implications for practice.
Prophylactic topical application of emollient ointment, particularly for very preterm infants in high‐income countries, has not been shown to reduce the risk of infection or its associated morbidity or mortality, and may increase the risk of infection with coagulase‐negative staphylococci.
In low‐ and middle‐income countries, the available data provide low certainty evidence that topical emollients (either proprietary ointments or inexpensive vegetable oils) reduce the risk of infection (though not mortality). Some evidence exists that massage with vegetable oil results in higher rates of weight gain and linear growth but the effect on long‐term growth and development is unknown.
Implications for research.
Given the potential for this simple, low‐cost, and readily available intervention to reduce the huge burden of infectious morbidity and mortality in preterm infants, particularly in low‐ and middle‐income countries, further pragmatic randomised controlled trials are justified in order to improve the precision of the estimates of effect sizes. We are aware of one such trial awaiting analysis and reporting (Kumar 2020).
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2021 | New citation required and conclusions have changed | Conclusions changed |
| 7 May 2021 | New search has been performed | Expanded and revised review. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 24 July 2003 | New citation required and conclusions have changed | Substantive amendment |
| 24 July 2003 | New search has been performed | This review updates the previously published review titled "Emollient ointment for preventing infection in preterm infants", The Cochrane Library, Issue 3, 1998 (Soll 1998). The updated review includes data from two additional randomized trials (Pabst 1999, Edwards 2001). Additional outcomes are noted including fungal infection, patent ductus arteriosus, bronchopulmonary dysplasia, and chronic lung disease. Results and conclusions have changed with inclusion of two more randomized trials. |
Acknowledgements
We are grateful to the principal investigators of the included trials for providing unpublished data for inclusion in this review.
We thank Newton Opiyo and Toby Lasserson of the Cochrane Central Editorial Unit for editorial advice and guidance.
For the 2021 update, Carol Friesen, Information Specialist, designed and ran the literature searches, and Colleen Ovelman peer reviewed the Ovid MEDLINE search strategy.
Jon Dorling and Robert Boyle have peer reviewed the 2021 review update.
Appendices
Appendix 1. 2021 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist; please see the Search Methodology section at https://neonatal.cochrane.org/resources-authors/author-resources-new-reviews.
CENTRAL via CRS Web:
Date ranges: 01 January 2015 to 08 January 2021 Terms: 1 MESH DESCRIPTOR Emollients EXPLODE ALL AND CENTRAL:TARGET 2 emollient* AND CENTRAL:TARGET 3 MESH DESCRIPTOR Skin Cream EXPLODE ALL AND CENTRAL:TARGET 4 skin cream* AND CENTRAL:TARGET 5 MESH DESCRIPTOR Ointments EXPLODE ALL AND CENTRAL:TARGET 6 MESH DESCRIPTOR Dermatologic Agents EXPLODE ALL AND CENTRAL:TARGET 7 dermatological agent* AND CENTRAL:TARGET 8 MESH DESCRIPTOR Plant Oils EXPLODE ALL AND CENTRAL:TARGET 9 MESH DESCRIPTOR Coconut Oil EXPLODE ALL AND CENTRAL:TARGET 10 MESH DESCRIPTOR Lanolin EXPLODE ALL AND CENTRAL:TARGET 11 lanolin AND CENTRAL:TARGET 12 MESH DESCRIPTOR Mineral Oil EXPLODE ALL AND CENTRAL:TARGET 13 MESH DESCRIPTOR Olive Oil EXPLODE ALL AND CENTRAL:TARGET 14 MESH DESCRIPTOR Petrolatum EXPLODE ALL AND CENTRAL:TARGET 15 petrolatum or petroleum jelly AND CENTRAL:TARGET 16 MESH DESCRIPTOR Soybean Oil EXPLODE ALL AND CENTRAL:TARGET 17 MESH DESCRIPTOR Sunflower Oil EXPLODE ALL AND CENTRAL:TARGET 18 ((plant* or vegetable* or coconut* or mineral* or olive* or soy or soybean* or sunflower* or almond*) ADJ3 oil*) AND CENTRAL:TARGET 19 aquaphor AND CENTRAL:TARGET 20 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 21 MESH DESCRIPTOR Skin EXPLODE ALL AND CENTRAL:TARGET 22 skin AND CENTRAL:TARGET 23 MESH DESCRIPTOR Administration, Cutaneous EXPLODE ALL AND CENTRAL:TARGET 24 cutaneous* AND CENTRAL:TARGET 25 MESH DESCRIPTOR Administration, Topical EXPLODE ALL AND CENTRAL:TARGET 26 topical* AND CENTRAL:TARGET 27 MESH DESCRIPTOR Skin Care EXPLODE ALL AND CENTRAL:TARGET 28 MESH DESCRIPTOR Skin Diseases, Bacterial EXPLODE ALL AND CENTRAL:TARGET 29 MESH DESCRIPTOR Skin Diseases EXPLODE ALL AND CENTRAL:TARGET 30 MESH DESCRIPTOR Massage EXPLODE ALL AND CENTRAL:TARGET 31 massage AND CENTRAL:TARGET 32 MESH DESCRIPTOR Skin Absorption EXPLODE ALL AND CENTRAL:TARGET 33 MESH DESCRIPTOR Skin Physiological Phenomena EXPLODE ALL AND CENTRAL:TARGET 34 #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 35 #34 AND #20 36 ((skin* or topical* or dermal or epidermal or cutaneous) ADJ6 (cream* or oil* or unguent* or gel* or moisturi* or honey or humectant* or ointment* or foam* or lotion* or conditioner*)) AND CENTRAL:TARGET 37 (topical* ADJ2 (agent* or treatment* or therap*)) AND CENTRAL:TARGET 38 skin care product* AND CENTRAL:TARGET 39 #35 OR #36 OR #37 OR #38 40 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 41 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 42 #41 OR #40 43 #42 AND #39 44 2015 TO 2021:YR AND CENTRAL:TARGET 45 #44 AND #43
MEDLINE via Ovid ‐ Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R):
Date ranges: 01 January 2015 to 08 January 2021 Terms: 1. exp Emollients/ 2. emollient*.mp. 3. exp Skin Cream/ 4. skin cream*.mp. 5. exp Ointments/ 6. exp Dermatologic Agents/ 7. dermatological agent*.mp. 8. exp Plant Oils/ 9. exp Coconut Oil/ 10. exp Lanolin/ 11. lanolin.mp. 12. exp Mineral Oil/ 13. exp Olive Oil/ 14. exp Petrolatum/ 15. (petrolatum or petroleum jelly).mp. 16. exp Soybean Oil/ 17. exp Sunflower Oil/ 18. ((plant* or vegetable* or coconut* or mineral* or olive* or soy or soybean* or sunflower* or almond*) adj3 oil*).mp. 19. aquaphor.mp. 20. or/1‐19 21. exp Skin/ 22. skin.mp. 23. exp Administration, Cutaneous/ 24. cutaneous*.mp. 25. exp Administration, Topical/ 26. topical*.mp. 27. exp Skin Care/ 28. exp Skin Diseases, Bacterial/ 29. exp Skin Diseases/ 30. exp Massage/ 31. massage.mp. 32. exp Skin Absorption/ 33. exp Skin Physiological Phenomena/ 34. or/21‐33 35. 20 and 34 36. ((skin* or topical* or dermal or epidermal or cutaneous) adj6 (cream* or oil* or unguent* or gel* or moisturi* or honey or humectant* or ointment* or foam* or lotion* or conditioner*)).mp. 37. (topical* adj2 (agent* or treatment* or therap*)).mp. 38. skin care product*.mp. 39. or/35‐38 40. exp infant, newborn/ 41. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 42. 40 or 41 43. randomized controlled trial.pt. 44. controlled clinical trial.pt. 45. randomized.ab. 46. placebo.ab. 47. drug therapy.fs. 48. randomly.ab. 49. trial.ab. 50. groups.ab. 51. or/43‐50 52. exp animals/ not humans.sh. 53. 51 not 52 54. 42 and 53 55. randomi?ed.ti,ab. 56. randomly.ti,ab. 57. trial.ti,ab. 58. groups.ti,ab. 59. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 60. placebo*.ti,ab. 61. 55 or 56 or 57 or 58 or 59 or 60 62. 41 and 61 63. limit 62 to yr="2019 ‐Current" 64. 54 or 63 65. 39 and 64 66. limit 65 to yr="2015 ‐Current"
ISRCTN:
Date searched: 2015 to 08 January 2021 Terms: Interventions: Topical emollient AND Participant age range: Neonate topical emollient within Participant age range: Neonate skin within Interventions: Emollient AND Participant age range: Neonate Condition: Infection AND Interventions: Skin* or topical* or dermal or epidermal or cutaneous AND Participant age range: Neonate skin* or topical* or dermal or epidermal or cutaneous AND ( Condition: Infection AND Participant age range: Neonate ) "skin* or topical* or dermal or epidermal or cutaneous AND ( Interventions: Lotion AND Participant age range: Neonate ) skin infection AND ( Interventions: Lotion AND Participant age range: Neonate ) skin within Interventions: Lotion AND Participant age range: Neonate
Appendix 2. Previous Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 7, 2015), MEDLINE (1966 to August 2015), EMBASE (1980 to August 2015, CINAHL (1982 to August 2015), and LILACS (1982 to August 2014) using the following text words and MeSH terms:
[exp Infant, Newborn/ OR Premature Birth/ OR (neonat$ or neo nat$).ti,ab. OR (newborn$ or new born$ or newly born$).ti,ab. OR (preterm or preterms or pre term or pre terms).ti,ab. OR (preemie$ or premie or premies).ti,ab. OR (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. OR (low adj3 (birthweight$ or birth weight$)).ti,ab. OR (lbw or vlbw or elbw).ti,ab. OR infan$.ti,ab. OR (baby or babies).ti,ab.] AND [emollients/ OR Skin cream/ OR Ointments/ OR Dermatological agents/ OR Plant oils/ OR emollient$.ti,ab. (skin adj6 (cream$ or oil$ or unguent$ or gel$ or moisturi$ or honey or humectant$ or ointment$ or foam$ or lotion$ or conditioner$)).ti,ab. OR (topical adj2 (agent$ or treatment$ or therap$)).ti,ab. OR skin care product$.ti,ab.]
The search outputs were limited with the relevant search filters for clinical trials. We did not apply any language restriction.
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/) and ClinicalTrials.gov (www.clinicaltrials.gov/) for completed or ongoing trials.
We examined reference lists in previous reviews and included studies. We searched the proceedings of the annual meetings of the Pediatric Academic Societies (1993 to present), the European Society for Paediatric Research (1995 to 2014), the Royal College of Paediatrics and Child Health (2000 to 2015), the Perinatal Society of Australia and New Zealand (2000 to 2015), the European Society for Paediatric Infectious Diseases (2005 to 2014), and the Infectious Diseases Society of America (2003 to 2014). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Appendix 3. 'Risk of bias' tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared pre‐specified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Data and analyses
Comparison 1. Topical ointment or cream versus routine skin care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Invasive infection (any organism) | 8 | 2086 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.97, 1.31] |
| 1.1.1 Low or middle income | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.28] |
| 1.1.2 High income | 6 | 1551 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
| 1.2 Invasive infection (trials with only very preterm infants participating) | 2 | 1210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.04, 1.50] |
| 1.3 Invasive infection (coagulase negative staphylococci) | 6 | 1839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.03, 1.65] |
| 1.3.1 Low or middle income | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.71, 2.22] |
| 1.3.2 High income | 4 | 1304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.02, 1.70] |
| 1.4 Invasive infection (other bacteria) | 6 | 1839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 1.4.1 Low or middle income | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.18] |
| 1.4.2 High income | 4 | 1304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| 1.5 Invasive infection (fungi) | 6 | 1839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.78, 2.06] |
| 1.5.1 Low or middle income | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.46, 6.65] |
| 1.5.2 High income | 4 | 1304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.72, 2.02] |
| 1.6 Mortality | 7 | 2067 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.03] |
| 1.6.1 Low or middle income | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 1.6.2 High income | 5 | 1532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.31] |
| 1.7 Mortality (trials with only very preterm infants participating) | 1 | 1191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.23] |
| 1.8 BPD | 2 | 1009 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| 1.9 NEC | 4 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 1.10 ROP (severe) | 1 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.28] |
Comparison 2. Topical oil versus routine skin care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Invasive infection (any organism) | 9 | 3256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.96] |
| 2.1.1 Low or middle income | 7 | 3162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.54, 0.99] |
| 2.1.2 High income | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 1.93] |
| 2.2 Invasive infection (coagulase negative staphylococci) | 7 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.02] |
| 2.2.1 Low or middle income | 5 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.16] |
| 2.2.2 High income | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.27] |
| 2.3 Invasive infection (other bacteria) | 7 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.04] |
| 2.3.1 Low or middle income | 5 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.05] |
| 2.3.2 High income | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.75] |
| 2.4 Invasive infection (fungi) | 7 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.42, 8.78] |
| 2.4.1 Low or middle income | 5 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.42, 8.78] |
| 2.4.2 High income | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.5 Mortality | 11 | 1119 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 2.5.1 Low or middle income | 8 | 976 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.82, 1.09] |
| 2.5.2 High income | 3 | 143 | Risk Ratio (IV, Fixed, 95% CI) | 0.11 [0.01, 1.99] |
| 2.6 Growth | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Rate of weight gain (g/kg/day) | 7 | 433 | Mean Difference (IV, Fixed, 95% CI) | 2.93 [2.11, 3.76] |
| 2.6.2 Change in crown‐heel length (mm/week) | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | 1.34 [0.20, 2.47] |
| 2.6.3 Change in head circumference (mm/week) | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐0.54, 1.85] |
| 2.6.4 Change in triceps skinfold thickness (mm/week) | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.03, 0.11] |
| 2.7 Moderate‐severe neurodevelopmental delay | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 BSID III cognitive score (<85) | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| 2.7.2 BSID III language score (<85) | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.21, 1.11] |
| 2.7.3 BSID III motor score (<85) | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| 2.7.4 Socio‐emotional score (<85) | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.07, 1.33] |
| 2.8 BPD | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.64] |
| 2.9 NEC | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.03] |
| 2.10 ROP (severe) | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.27, 3.69] |
| 2.11 Severe neurodevelopmental disability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.11.1 BSID (3rd Ed): Cognitive <70 | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.60] |
| 2.11.2 BSID (3rd Ed): Language <70 | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.95] |
| 2.11.3 BSID (3rd Ed): Motor <70 | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.42] |
| 2.11.4 BSID (3rd Ed): Social‐emotional <70 | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.54] |
2.11. Analysis.

Comparison 2: Topical oil versus routine skin care, Outcome 11: Severe neurodevelopmental disability
Comparison 3. Topical ointment or cream vs. topical oil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Invasive infection (any organism) | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.57, 1.46] |
| 3.2 Invasive infection (coagulase negative staphylococci) | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Invasive infection (other bacteria) | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.50] |
| 3.4 Invasive infection (fungi) | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.31, 5.94] |
| 3.5 Mortality | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.98] |
Comparison 4. One topical oil (or combination) vs. another oil (or combination).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Invasive infection | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.2 Mortality | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3 Growth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 Rate of weight gain (g/kg/day) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [0.84, 3.16] |
| 4.3.2 Change in crown‐heel length (mm/week) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.29, 1.09] |
| 4.3.3 Change in head circumference (mm/week) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.17, 0.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alkharfy 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Infants weighing 501 grams to 1250 grams at birth and with a gestational age of < 33 weeks. | |
| Interventions | 1. Twice‐daily topical therapy of 2 g/kg petroleum jelly (N = 35) 2. Standard skin care (N = 39) Intervention applied until 34 weeks' postmenstrual age. |
|
| Outcomes | Invasive infection. NEC. BPD. Mortality. |
|
| Notes | Setting: Department of Pediatrics, King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia (January 2008 to December 2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes kept in closed closet, managed by study co‐ordinator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Arora 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants of birth weight < 1500 grams, and < 10 days old, who were receiving enteral feeds. | |
| Interventions | 1. Massage with sunflower oil: N = 23 2. Massage without oil: N = 23 3. No massage (or oil): N = 23 Intervention applied for at least 10 days after enrolment. We combined groups 2 and 3 as a single control group for meta‐analyses. |
|
| Outcomes | Growth. Invasive infection. Mortality. | |
| Notes | Setting: Regional Neonatal Unit, Associated Lok Nayak Hospital, New Dehli, India (March to December 2001). Infection and mortality data courtesy of Professor Kumar. SD for change in length and head circumference imputed from Soriano 2000. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete follow‐up assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Darmstadt 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants of gestational age < 34 weeks and postnatal age < 72 hours. Exclusions: Infants considered likely to die within 48 hours, infants with major congenital anomalies, infants requiring major surgery, infants with immunodeficiency. |
|
| Interventions | 1. Cutaneous application of sunflower seed oil to whole body apart from face and head (4 grams/kg/dose): N = 51. Infants received emollient thrice daily for 14 days, then twice daily until 28 days or discharge from hospital. 2. No emollient: N = 52. Control infants received standard skin care for preterm infants, which included minimal to no use of topical emollients. |
|
| Outcomes | Mortality attributed to sepsis. Invasive infection. |
|
| Notes | Setting: Kasr El‐Aini NICU, Cairo University (dates not stated but trial likely to have been undertaken during late 1990s or early 2000s). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | Mortality attributed to sepsis (all‐cause mortality not reported or available from investigators). Invasive infection diagnosed more than 2 days after birth: the report did not state the number of infants in each group who had (at least one) episode of invasive infection. We contacted the principal investigator to seek these data but these have not yet been provided. |
| Other bias | Low risk | No evidence of baseline imbalance |
Darmstadt 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants of gestational age at birth < 33 weeks and aged < 72 hours. Exclusions: Infants considered likely to die within 48 hours, infants with major congenital anomalies, infants requiring major surgery, infants with established skin infections. |
|
| Interventions | Massage (whole body apart from face and head) thrice daily for 14 days, then twice daily until discharge from hospital with: 1. Sunflower seed oil: N = 159 2. Aquaphor: N = 157 3. No emollient (control): N = 181 |
|
| Outcomes | Invasive infection. Mortality. | |
| Notes | Setting: Special Care Nursery, Dhaka Shishu Hospital, Bangladesh (1998 to 2003). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Manual generation of blocks of six with two assignments per block for all three of the groups, then computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete follow‐up assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Unclear risk | The report states that infants were randomised within "blocks of six with two assignments per block for all three of the groups". However, this process appears to be inconsistent with the allocated distribution of infants: 1. sunflower seed oil (N = 159), or 2. Aquaphor (N = 157), or 3. no emollient (control) (N = 181). The principal investigator of the trial has explained that this inconsistency may be due to two possible factors:
1. The trial originally had a fourth arm (emollient therapy with safflower oil) which was deemed unacceptable to parents and caregivers and removed from the trial when 24 infants had been randomised to the arms. These infants were not included in the final analyses. 2. The allocation sequence generation method was changed from the original manual process (selecting from a block of six envelopes) to a computer‐generated sequence that did not maintain the balance of assignments within blocks. |
Edwards 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants, < 31 weeks gestation and birth weight 501 g to 1000 g, aged < 48 hours without evidence of skin disease and expected to survive beyond 48 hours. | |
| Interventions | 1. Prophylactic application of preservative free ointment (Aquaphor, Beiersdorf Inc.) twice daily: N = 602 2. Routine skin care: N = 589 (could include local application of Aquaphor to area of dermatitis if required) Intervention applied for 14 days. |
|
| Outcomes | Invasive infection.
Mortality. BPD. NEC. ROP. |
|
| Notes | 53 centres across Vermont Oxford Network in USA (trial performed during late 1990s to early 2000s). Dr Soll provided information on randomisation method and data on fungal infections. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near complete outcome assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Erdemir 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants (< 34 weeks) and < 24 hours old. Exclusions: Admitted after 24 hours, major congential abnormalities, infection (skin or systemic). |
|
| Interventions | 1. Aquaphor once daily to entire body surface except head: N = 100 2. Routine skin care without emollient: N = 97 Intervention applied for 14 days. |
|
| Outcomes | Infection. Mortality. NEC. |
|
| Notes | Setting: Tepecik Hospital, Turkey (2010 to 2012). Additional information courtesy of investigators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Fallah 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Newborn infants (< 10 days old) of gestational age 33 to 37 weeks, and birth weight 1500 g to 1999 g. Exclusions: multiple pregnancy, birth asphyxia, sepsis, major congenital malformation, small for gestational age. |
|
| Interventions | 1. Massage with sunflower oil, three times daily (N = 30) 2. Massage without oil (N = 30) Intervention applied for 14 days. |
|
| Outcomes | Growth parameters. Mortality. Infection data not available from investigators (May 2014). |
|
| Notes | Setting: Shahid Saddoughi Hospital, Yazd, Iran (2011). Growth parameters reported as mean at baseline and end of intervention. We calculated mean change and imputed the associated SD for weight gain from Kumar 2013 and SD for change in length and head circumference from Soriano 2000 (similar population, and same timescale of measurement). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete follow‐up: 5 infants (2 in intervention group, and 3 controls) were lost to follow‐up (and growth parameter data not available). |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Farhat 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (< 37 weeks) or birth weight < 2000 g and receiving 150 mL/kg/day of breast milk | |
| Interventions | 1. Daily massage with sunflower oil: N = 30 2. Routine skin care (no massage): N = 29 Intervention applied for seven days. |
|
| Outcomes | Weight change [mean estimated from graph, SD imputed from Kumar 2013] | |
| Notes | Setting: Emamreza Hospital, Mashhad, Iran (2007‐9). Further data on infection and mortality not available from investigators (May 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete assessment of weight change outcome |
| Selective reporting (reporting bias) | Unclear risk | Data on infection and mortality not reported or available from investigators (May 2014). |
| Other bias | Low risk | No evidence of baseline imbalance |
Jabraeile 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants 28 to 32 weeks' gestation, or birth weight 1000 g to 1500 g. Exclusions: infants with congenital anomalies, or signs of physiological instability. |
|
| Interventions | 1. Olive oil massage thrice daily: N = 45 2. Massage without emollient: N = 45 Intervention applied for up to 10 days. |
|
| Outcomes | Duration of hospitalisation. Weight gain during the 10 days intervention period. |
|
| Notes | Setting: Neonatal Intensive Care Unit, Al‐Zahra Hospital, Tabriz, Iran (2018). The mean weight at baseline differed between the groups (1321 g versus 1145 g; MD 176 g, 95% CI 119 to 233). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | High risk | Investigators likely to have been prospectively aware of group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (86/90) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Kanti 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (< 48 hours after birth) with birth weight 1500 grams to 2500 grams | |
| Interventions | 1. Sunflower seed oil daily applied to whole body every 3 to 4 hours for 10 days: N = 11 2. Control (no oil): N = 11 |
|
| Outcomes | Transepidermal water loss, stratum corneum hydration, skin pH and sebum level (no episodes of invasive infection or death) | |
| Notes | Setting: Department of Neonatology, Charité Universitätsmedizin Berlin, Germany (2009 to 2011). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment |
| Selective reporting (reporting bias) | Unclear risk | Data on clinical outcomes courtesy of Dr Bartels (2015). |
| Other bias | Low risk | No evidence of baseline imbalance |
Kiechl‐Kohlendorfer 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (25 to 36 weeks) admitted to NICU (neonatal intensive care unit) | |
| Interventions | 1. Water‐in‐oil emollient cream (Bepanthen): N = 57 2. Olive oil cream (70% lanolin, 30% olive oil): N = 58 3. Routine skin care (control group): N = 58 Emollients applied twice daily to body surface except head for 4 weeks |
|
| Outcomes | Invasive infection. Mortality. |
|
| Notes | Setting: Department of Pediatrics, Innsbruck Medical University, Innsbruck, Austria (2004 to 2006). We combined groups 1 and 2 as a single intervention (ointment) group for meta‐analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Konar 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants < 37 weeks (modified Ballard scoring) Exclusions: infants with congenital anomalies, skin rash or infection, critically ill. |
|
| Interventions | 1. Coconut oil (5 mL) massaged on entire body except face and scalp four times daily: N = 1146 2. Massage only (no emollient): N = 1148 Intervention applied for seven days. |
|
| Outcomes | Skin condition during neonatal period. Neurodevelopmental score (motor/mental) in infancy Invasive infection (> 72 h after birth) defined as "clinical signs with positive sepsis screen with or without positive blood culture". |
|
| Notes | Setting: "Rural field practice", Department of Community Medicine, Burdwan Medical College, India (2014 to 2018). Unpublished data on infants with positive blood culture supplied by Dr Konar. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Kukreja 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants with birth weight 1000 g to 2000 g. Exclusions: infants with major congenital anomalies, skin disease, need for mechanical ventilation for >12 h. |
|
| Interventions | 1. Topical sunflower seed oil (4 mL/kg) applied to entire body below neck thrice daily: N = 39 2. Standard skin care (no emollient): N = 39 Intervention applied for seven days. |
|
| Outcomes | Invasive infection (until day 28). Skin condition and microbial colonisation. |
|
| Notes | Setting: Neonatal Unit, Maulana Azad Medical College, New Dehli, India (2015 to 2016). Mortality data courtesy of Prof Ajay Kumar (February 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Serial, sealed, opaque, envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Kumar 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants of birth weight < 1800 grams and < 35 weeks' gestation, who were receiving ≥ 100 ml/kg/day enteral feeds. Exclusions: Infants receiving supplemental oxygen > 48 hours after birth, infants with major congenital anomalies, intracranial haemorrhage, meningitis or encephalopathy. |
|
| Interventions | 1. Massage with sunflower oil four times daily: N = 27 2. Standard care (no massage): N = 25 Intervention applied for up to 28 days. |
|
| Outcomes | Growth parameters. Mortality. |
|
| Notes | Setting: Neonatal Intensive Care Unit (NICU), LLRM Medical College, Meerut, India (2009 to 2010). Cross‐over contamination was limited by "segregating the two groups in separate NICU rooms". SD for change in length and head circumference imputed from Soriano 2000. Data on invasive infection not available from investigators (May 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete growth data assessment (3 randomised infants not included because of protocol violation or loss to follow‐up) |
| Selective reporting (reporting bias) | Unclear risk | Data on invasive infection not available from investigators |
| Other bias | Low risk | No evidence of baseline imbalance |
Lane 1993.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (gestational age 29 to 36 weeks), aged < 24 hours. | |
| Interventions | 1. Prophylactic application of water‐in‐oil emollient (Eucerin Creme, Beiersdorf, Inc.) twice daily: N = 17
2. No emollient (standard skin care): N = 17 Intervention applied for 16 days. |
|
| Outcomes | Invasive infection. Mortality (no deaths). |
|
| Notes | Setting: Departments of Dermatology and Pediatrics, Stanford University, California, early 1990s (single centre). Clarification of methods and outcomes courtesy of Professor Alfred Lane (May 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Nopper 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (gestational age < 33 weeks), aged < 96 hours, without evidence of skin disease. | |
| Interventions | 1. Prophylactic application of preservative‐free ointment (Aquaphor, Beiersdorf, Inc.) applied twice daily: N = 30
2. Standard skin care (which could include a water‐in‐oil emollient if required (Eucerin, Beiersdorf, Inc.): N = 30 Intervention applied for 14 days. |
|
| Outcomes | Invasive infection. Mortality. Weight change. Time to regain birth weight. |
|
| Notes | Setting: Departments of Dermatology and Pediatrics, Stanford University, California, early‐mid 1990s (single centre). Clarification of methods and outcomes courtesy of Professor Alfred Lane (May 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Pabst 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (gestational age 26 to 30 weeks), aged < 24 hours. | |
| Interventions | 1. Prophylactic application of preservative‐free ointment (Aquaphor Ointment, Beiersdorf, Inc.) applied twice daily: N = 11
2. Standard skin care (no emollients): N = 8 Intervention applied for 14 days. |
|
| Outcomes | Weight change. Invasive infection. [Mortality not reported] |
|
| Notes | Setting: Department of Pediatrics, University of Maryland, Baltimore, US (mid‐1990s). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Salam 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Hospital‐born preterm infants (gestational age > 26 weeks). | |
| Interventions | 1. Coconut oil massage twice daily: N = 128 2. Routine skin care: N = 130 Twice daily topical application of coconut oil by nurses from birth until discharge and continued thereafter by mothers at home until completion of the 28th day of life. |
|
| Outcomes | Invasive infection. Neonatal mortality. |
|
| Notes | Setting: Nursery and neonatal intensive care unit at Aga Khan University Hospital, Pakistan. ClinicalTrials.gov Identifier: NCT01396642 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Staff "blinded to randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Sankaranarayanan 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Appropriate for gestational age preterm infants with birth weight 1500 g to 2000 g | |
| Interventions | 1. Coconut oil massage: N = 38 2. Mineral oil massage: N = 37 3. Massage with baby powder: N = 37 Intervention applied from day 2 until day 31 after birth (4 times daily for 5 minutes). |
|
| Outcomes | Growth parameters. | |
| Notes | Setting: Department of Neonatology, LTM Medical College and General Hospital, Mumbai, India. Infection and mortality data courtesy of Dr Sankaranarayanan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Soriano 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants (gestational age 28 to 34 weeks and birth weight < 1700 grams). Infants were fully enterally fed before entering the trial. Exclusions: Infants receiving supplemental oxygen, ventilation, inotropic agents, corticosteroids, and infants with congenital anomalies. |
|
| Interventions | 1. Thrice daily cutaneous application of soybean oil (6 grams/kg/day of linoleic acid) for 30 days: N = 29 2. No cutaneous treatment: N = 31 |
|
| Outcomes | Growth parameters. Invasive infection. Mortality. | |
| Notes | Setting: Newborn Nursery, Hospital das Clinicas de Ribeirao Preto, Sao Paulo, Brazil (1992 to 1993). Further data courtesy of Dr Francisco Martinez. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome assessment |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Strunk 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | Preterm infants < 30 weeks' gestation, postnatal age < 24 hours. Exclusions: infants with major congenital anomalies, or signs of pre‐existing skin infection. |
|
| Interventions | 1. Topical coconut oil (5 mL/kg) twice daily: N = 36 2. Standard skin care (no emollient): N = 36 Intervention applied for up to 21 days. |
|
| Outcomes | Skin condition until day 21. Weight gain. Invasive infection. Necrotising enterocolitis (NEC). Mortality. Cognitive, language, motor or socio‐emotional delay assessed at 24 months (corrected age) using Bayley Scales of Infant Development (3rd Edition). |
|
| Notes | Setting: Neonatal Intensive Care Unit, Edward Memorial Hospital, Perth, Australia (2018). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed, coded, opaque, serially‐numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete for in hospital outcomes (25% attrition for neurodevelopmental assessments) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Vaivre‐Douret 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (31 to 34 weeks). Exclusions: Mechanical ventilation or supplemental oxygen requirement, maternal drug misuse, congenital abnormalities. |
|
| Interventions | 1. Almond oil or vegetable oil massage twice daily for ten days: N = 24 2. Saline massage or no intervention (routine care): N = 25 |
|
| Outcomes | Growth (weight and length percentage change) reported but data suitable for inclusion in meta‐analyses not available. Mortality (no deaths). Infection not reported and data not available from investigators. |
|
| Notes | Setting: Tertiary Neonatal Unit, Poitou‐Charentes, France (2002 to 2004). We have sought but have not received further clarification and data from the investigators (May 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | High risk | Clinician allocation (likely to have been aware) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked parents and caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked clinicians and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete growth parameters data reported |
| Selective reporting (reporting bias) | Unclear risk | Infection not reported and data not available from investigators |
| Other bias | Low risk | No evidence of baseline imbalance |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdallah 2013 | Quasi‐experimental design (epoch‐comparison). |
| Ang 2012 | No emollient in intervention. |
| Beeram 2006 | Retrospective case control study. |
| Berger 2009 | Duplicate. Article reporting same research as Kiechl‐Kohlendorfer 2008. |
| Brandon 2010 | No Sting (not an emollient) versus emollient; no routine care as control arm. |
| Brice 1981 | Comparison of paraffin mixture with thermal blankets. |
| Caglar 2020 | Randomised controlled trial. Preterm infants 32‐ 37 weeks' gestation. Measured effect of topical sunflower oil versus almond oil versus no emollient on skin condition and hydration over first five days. No data on outcome measures of interest. |
| Campbell 2000 | Case control study. |
| Diego 2008 | No emollient in intervention. |
| Ferber 2005 | No emollient in intervention. |
| Fernandez 1987 | Case control study. |
| Fernandez 2005 | Not a randomised controlled trial. |
| Guzzetta 2011 | No emollient in intervention. |
| Jansi 2008 | Partcipants predominantly term babies ‐ outcomes for preterm infants not reported. |
| Mathai 2001 | Quasi‐randomised trial of massage using powder or mineral oil ‐ no routine care as control arm. |
| Mendes 2008 | No emollient in intervention. |
| Montaseri 2020 | Randomised controlled trial. Preterm infants 30 to 36 weeks' gestation. Measured effect of massage with olive oil versus no emollient on weight change after first five days. No data on outcome measures of interest. Irct20170520034039N |
| Rutter 1981 | Non‐random: "before‐and‐after" (epoch‐comparison) study. |
| Smith 2013 | No emollient in intervention. |
| Solanki 2005a | Randomised controlled trial. Measured effect of topical safflower oil versus coconut oil versus no oil on fatty acid profiles and triglyceride levels in blood. No data on outcome measures of interest. |
| Taheri 2018 | Randomised controlled trial. Assessed effect of sunflower oil massage on weight change over first five days after birth. No data on outcome measures of interest. |
| Wananukul 2001 | Comparison of topical application of liquid paraffin mixture to one side of the body with no application to the other side. |
| Wananukul 2002 | Effect of liquid paraffin emollient on trans‐epidermal water loss and ambient skin temperature. Outcomes measured for 5 hours only. |
Characteristics of studies awaiting classification [ordered by study ID]
Hu 2014.
| Methods | Randomised controlled trial |
| Participants | 428 preterm infants < 34 weeks' gestational age and < 72 hours old were considered eligible for the study during September 2010 to June 2012 |
| Interventions | Infants were randomly assigned to sunflower seed oil group (153 cases) or Johnson oil group (140 cases) or control group (135 cases) |
| Outcomes | Skin condition Infection |
| Notes |
Maamouri 2018.
| Methods | Unclear whether this is an RCT: "The subjects were placed in two groups" (clarification sought from investigators) |
| Participants | 60 preterm infants (< 34 weeks) |
| Interventions | 1. Sunflower oil (N = 27) 2. Control group (N = 33) The body surface of these infants was massaged with sunflower oil three times a day for 2 weeks and then twice for up to 28 days. |
| Outcomes | Skin condition Blood culture‐confirmed infection |
| Notes | Setting: Neonatal Intensive Care Unit, Ghaem Hospital in Mashhad, Iran (2016): Irct20130120012197N |
Nangia 2015.
| Methods | Randomised controlled trial |
| Participants | Preterm infants with birth weight 751 g to 1499 g. Exclusions: infants with major congenital anomalies or asphyxia or hydrops. |
| Interventions | 1. Topical coconut oil (4 mL) applied to trunk below neck twice daily: N = 37 2. Standard skin care (no emollient): N = 37 Intervention applied for seven days. |
| Outcomes | Trans‐epidermal water loss. Microbial skin colonisation after seven days. |
| Notes | Setting: Neonatal Unit, Kalawati Saran Children's Hospital, New Dehli, India (2006). Data on invasive infection and mortality sought March 2021. |
Saeidi 2014.
| Methods | Randomised controlled trial. |
| Participants | Preterm infants < 37 weeks' gestation, postnatal age < 28 days. Exclusions: infants with major congenital anomalies, pre‐existing skin conditions, or receiving respiratory support. |
| Interventions | 1. Massage with medium chain triglyceride (likely coconut) oil (4 mL) applied below neck four times daily: N = 40 2. Massage only (no emollient): N = 41 Intervention applied for seven days. |
| Outcomes | Weight change until day 7. |
| Notes | Setting: Neonatal Intensive Care Unit, Qaem Educational Hospital, Mashhad, Iran (trial period not reported). Data on invasive infection and mortality sought March 2021. |
Summers 2019.
| Methods | Cluster randomised controlled trial. |
| Participants | Preterm infants of gestational age at birth < 37 weeks Exclusions: Infants considered likely to die within 48 hours, infants with major congenital anomalies, infants requiring major surgery, infants with established skin infections. |
| Interventions | "Full‐body" massage with: 1. Sunflower seed oil: N = 195 2. Mustard oil: N = 204 Promoted daily massage by mothers for 28 days. |
| Outcomes | Trans‐epidermal water loss. Skin condition and pH. |
| Notes | Community‐based; Sarlahi, Nepal (2012 to 2014) Data on invasive infection and mortality sought March 2021. |
Characteristics of ongoing studies [ordered by study ID]
Kumar 2020.
| Study name | Impact of topical application of cold‐pressed sunflower seed oil with improved massage practices on neonatal mortality: a cluster‐randomised controlled trial in rural North India |
| Methods | Cluster‐randomised controlled trial |
| Participants | All newborns identified within the study area until 7 days after their delivery are considered eligible for the trial |
| Interventions | 1. Sunflower seed oil massage (10 mL, three times daily) by families through the neonatal period 2. Usual care |
| Outcomes | Neonatal mortality rate after 24 hours of birth. |
| Starting date | 01/01/2015 |
| Contact information | Dr Vishwajeet Kumar, Uttar Pradesh, Lucknow, India (vishwajeet.kumar@shivgarh.org) |
| Notes | ISRCTN38965585 WHO Reference No.: RPC667 The Universal Trial Number (UTN): U1111‐1158‐4665 [subgroup data for preterm infants will be sought] |
Differences between protocol and review
Expanded and revised protocol and review, with pre‐specified analyses of trials conducted in low‐ and middle‐income versus high‐income countries. We planned sensitivity analyses to determine if the findings are affected by including only studies of adequate methodology (low risk of bias), defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up.
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL (Ovelman 2020).
Since July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from the World Health Organization’s International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN Registry (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
Starting in September 2020, Cochrane Neonatal no longer searches for RCTs and quasi‐RCTs from CINAHL, as records are identified and added to CENTRAL on a monthly basis through Cochrane's Centralised Search Service project (see 'How CENTRAL is created' at: www.cochranelibrary.com/central/central-creation#CINAHL%20section).
For the 2021 update, we ran searches in the following databases: CENTRAL via Cochrane Register of Studies (CRS) Web and MEDLINE via Ovid. The search strategies are available in Appendix 1. The previous search methods are available in Appendix 2.
Contributions of authors
Jemma Cleminson and William McGuire updated the search strategy, appraised the identified literature, extracted data from included studies and drafted the revised review.
Sources of support
Internal sources
-
Centre for Reviews and Dissemination, University of York, UK
Logistical
External sources
-
National Institute of Health Research (NIHR), UK
This report is independent research funded by a UK NIHR Evidence Synthesis Programme Grant.
-
World Health Organization, Switzerland
Editorial and administrative support for this review has been provided by a grant from World Health Organization to Cochrane Neonatal.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alkharfy 2014 {published data only}
- AlKharfy T, Ba-Abbad R, Hadi A, Alfaleh K. Use of topical petroleum jelly for prevention of sepsis in very low-birthweight infants: a prospective, randomised controlled trial. Paediatrics and International Child Health 2014;34(3):194-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arora 2005 {published data only}
- Arora J, Kumar A, Ramji S. Effect of oil massage on growth and neurobehavior in very low birth weight preterm neonates. Indian Pediatrics 2005;42(11):1092-100. [PMID: ] [PubMed] [Google Scholar]
Darmstadt 2004 {published data only}
- Darmstadt GL, Badrawi N, Law PA, Ahmed S, Bashir M, Iskander I, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatric Infectious Disease Journal 2004;23(8):719-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Mao-Qiang M. Topical therapy to improve epidermal barrier function and prevent infections in preterm infants in developing countries. Pediatric Research 2003;53:316A. [Google Scholar]
Darmstadt 2005 {published data only}
- Ahmed AS, Saha SK, Chowdhury MA, Law PA, Black RE, Santosham M, et al. Acceptability of massage with skin barrier-enhancing emollients in young neonates in Bangladesh. Journal of Health, Population, and Nutrition 2007;25(2):236-40. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Ahmed S, Ahmed AN, Saha SK. Mechanism for prevention of infection In preterm neonates by topical emollients: a randomized, controlled clinical trial. Pediatric Infectious Disease Journal 2014;33(11):1124-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Saha SK, Ahmed AS, Choi Y, Chowdhury MA, Islam M, et al. Effect of topical emollient treatment of preterm neonates in Bangladesh on invasion of pathogens into the bloodstream. Pediatric Research 2007;61(5 Pt 1):588-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Saha SK, Ahmed AS, Chowdhury MA, Law PA, Ahmed S, et al. Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet 2005;365(9464):1039-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Edwards 2004 {published data only}
- Edwards WH, Conner JM, Soll RF, Vermont Oxford Network Neonatal Skin Care Study Group. The effect of prophylactic ointment therapy on nosocomial sepsis rates and skin integrity in infants with birth weights of 501 to 1000 g. Pediatrics 2004;113(5):1195-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Edwards WH, Conner JM, Soll RF. The effect of Aquaphor Original Emollient Ointment on nosocomial sepsis rates and skin integrity in infants of birth weight 501 to 1000 grams. Pediatric Research 2001;49:388A. [DOI] [PubMed] [Google Scholar]
Erdemir 2015 {published data only}
- Erdemir A, Kahramaner Z, Yuksel Y, Cosar H, Turkoglu E, Sutcuoglu S, et al. The effect of topical ointment on neonatal sepsis In preterm infants. The Journal of Maternal-Fetal & Neonatal Medicine 2014;28(1):33-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fallah 2013 {published data only}
- Fallah R, Akhavan Karbasi S, Golestan M, Fromandi M. Sunflower oil versus no oil moderate pressure massage leads to greater increases in weight in preterm neonates who are low birth weight. Early Human Development 2013;89(9):769-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farhat 2010 {published data only}
- Farhat AS, Mohammadzadeh A, Amiri R, Amiri M. Effect of dermal sunflower oil on growth of low birth weight preterm infants. In: XXII European Congress of Perinatal Medicine; 2010 May 26-29; Granada, Spain. Granada, Spain, 2010.
Jabraeile 2016 {published data only}
- Jabraeile M, Rasooly As, Farshi Mr Malakouti J. Effect of olive oil massage on weight gain in preterm infants: A randomized controlled clinical trial. Nigerian Medical Journal 2016;57(3):160-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanti 2014 {published data only}
- Kanti V, Grande C, Stroux A, Buhrer C, Blume-Peytavi U, Garcia Bartels N. Influence of sunflower seed oil on the skin barrier function of preterm infants: a randomized controlled trial. Dermatology 2014;229(3):230-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kiechl‐Kohlendorfer 2008 {published data only}
- Berger C, Inzinger R. Study of skin care in premature and newborn infants [Studie zur Hautpflege bei Fruh- und Neugeborenen.]. Kinderkrankenschwester : Organ der Sektion Kinderkrankenpflege / Deutsche Gesellschaft fur Sozialpadiatrie und Deutsche Gesellschaft fur Kinderheilkunde 2009;28(3):116-25. [PubMed] [Google Scholar]
- Kiechl-Kohlendorfer U, Berger C, Inzinger R. The effect of daily treatment with an olive oil/lanolin emollient on skin integrity in preterm infants: a randomized controlled trial. Pediatric Dermatology 2008;25(2):174-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Konar 2019 {published data only}
- Konar M C, Islam K, Roy A, Ghosh T. Effect of virgin coconut oil application on the skin of preterm newborns: a randomized controlled trial. Journal of Tropical Pediatrics 2019;66(2):129-135. [DOI] [PubMed] [Google Scholar]
Kukreja 2018 {published data only}
- Kukreja B, Kumar A, Satyanarayana K. Effect on nosocomial sepsis of topical oil application, skin condition, and care practice device usage in preterm neonates: A randomized controlled trial. Indian Journal of Child Health 2018;5:689-693. [Google Scholar]
Kumar 2013 {published data only}
- Kumar J, Upadhyay A, Dwivedi AK, Gothwal S, Jaiswal V, Aggarwal S. Effect of oil massage on growth in preterm neonates less than 1800 g: A randomized control trial. Indian Journal of Pediatrics 2013;80(6):465-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lane 1993 {published data only}
- Lane AT, Drost SS. Effects of repeated application of emollient cream to premature neonates' skin. Pediatrics 1993;92(3):415-9. [PMID: ] [PubMed] [Google Scholar]
Nopper 1996 {published data only}
- Nopper AJ, Horii KA, Sookdeo-Drost S, Wang TH, Mancini AJ, Lane AT. Topical ointment therapy benefits premature infants. Journal of Pediatrics 1996;128(5 Pt 1):660-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pabst 1999 {published data only}
- Pabst RC, Starr KP, Qaiyumi S, Schwalbe RS, Gewolb IH. The effect of application of aquaphor on skin condition, fluid requirements, and bacterial colonization in very low birth weight infants. Journal of Perinatology 1999;19(4):278-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salam 2015 {published data only}
- Salam RA, Darmstadt GL, Bhutta ZA. Effect of emollient therapy on clinical outcomes in preterm neonates in Pakistan: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(3):F210-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Salam RA, Das JK, Darmstadt GL, Bhutta ZA. Emollient therapy for preterm newborn infants - evidence from the developing world. BMC Public Health 2013;13 Suppl 3:S31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankaranarayanan 2005 {published data only}
- Sankaranarayanan K, Mondkar JA, Chauhan MM, Mascarenhas BM, Mainkar AR, Salvi RY. Oil massage in neonates: an open randomized controlled study of coconut versus mineral oil. Indian Pediatrics 2005;42(9):877-84. [PMID: ] [PubMed] [Google Scholar]
Soriano 2000 {published data only}
- Soriano CR, Martinez FE, Jorge SM. Cutaneous application of vegetable oil as a coadjutant in the nutritional management of preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2000;31(4):387-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strunk 2018 {published data only}
- Strunk T, Nathan E, Sharp M, Doherty D, Patole S. Developmental outcomes following topical coconut oil in very preterm infants. Neonatology 2019;116(3):302-304. [DOI] [PubMed] [Google Scholar]
- Strunk T, Pupala S, Hibbert J, Doherty D, Patole S. Topical coconut oil in very preterm infants: an open-label randomised controlled trial. Neonatology 2018;113(2):146-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vaivre‐Douret 2008 {published data only}
- Vaivre-Douret L, Oriot D, Blossier P, Py A, Kasolter-Pere M, Zwang J. The effect of multimodal stimulation and cutaneous application of vegetable oils on neonatal development in preterm infants: a randomized controlled trial. Child: Care, Health and Development 2009;35(1):96-105. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdallah 2013 {published data only}
- Abdallah B, Badr LK, Hawwari M. The efficacy of massage on short and long term outcomes in preterm infants. Infant Behavior & Development 2013;36(4):662-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ang 2012 {published data only}
- Ang JY, Lua JL, Mathur A, Thomas R, Asmar BI, Savasan S, et al. A randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics 2012;130(6):e1549-58. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beeram 2006 {published data only}
- Beeram M, Olvera R, Krauss D, Loughran C, Petty M. Effects of topical emollient therapy on infants at or less than 27 weeks' gestation. Journal of the National Medical Association 2006;98(2):261-4. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Berger 2009 {published data only}
- Berger C, Inzinger R. Study of skin care in premature and newborn infants [Studie zur Hautpflege bei Fruh- und Neugeborenen.]. Kinderkrankenschwester : Organ der Sektion Kinderkrankenpflege / Deutsche Gesellschaft fur Sozialpadiatrie und Deutsche Gesellschaft fur Kinderheilkunde 2009;28(3):116-25. [PMID: ] [PubMed] [Google Scholar]
Brandon 2010 {published data only}
- Brandon DH, Coe K, Hudson-Barr D, Oliver T, Landerman LR. Effectiveness of No-Sting skin protectant and Aquaphor on water loss and skin integrity in premature infants. Journal of Perinatology 2010;30(6):414-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brice 1981 {published data only}
- Brice JE, Rutter N, Hull D. Reduction of skin water loss in the newborn. II. Clinical trial of two methods in very low birthweight babies. Archives of Disease in Childhood 1981;56(9):673-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caglar 2020 {published data only}
- Caglar S, Yildiz GK, Bakoglu I, Salihoglu O. The effect of sunflower seed and almond oil on preterm infant skin: a randomized controlled trial. Advances in Skin & Wound Care 2020;33(8):1-6. [DOI] [PubMed] [Google Scholar]
Campbell 2000 {published data only}
- Campbell JR, Zaccaria, E, Baker CJ. Systemic candidiasis in extremely low birth weight infants receiving topical petrolatum ointment for skin care: a case-control study. Pediatrics 2000;105(5):1041-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Diego 2008 {published data only}
- Diego MA, Field T, Hernandez-Reif M. Temperature increases in preterm infants during massage therapy. Infant Behavior & Development 2008;31(1):149-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferber 2005 {published data only}
- Ferber SG, Feldman R, Kohelet D, Kuint J, Dollberg S, Arbel E, et al. Massage therapy facilitates mother-infant interaction in premature infants. Infant Behavior & Development 2005;28(1):74-81. [Google Scholar]
Fernandez 1987 {published data only}
- Fernandez A, Patkar S, Chawla C, Taskar T, Prabhu SV. Oil application in preterm babies--a source of warmth and nutrition. Indian Pediatrics 1987;24(12):1111-6. [PMID: ] [PubMed] [Google Scholar]
Fernandez 2005 {published data only}
- Fernandez AR, Krishnamoorthy G, Patil N, Mondkar JA, Swar BD. Transcutaneous absorption of oil in preterm babies--a pilot study. Indian Pediatrics 2005;42(3):255-8. [PMID: ] [PubMed] [Google Scholar]
Guzzetta 2011 {published data only}
- Guzzetta A, D'Acunto MG, Carotenuto M, Berardi N, Bancale A, Biagioni E, et al. The effects of preterm infant massage on brain electrical activity. Developmental Medicine and Child Neurology 2011;53 Suppl 4:46-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jansi 2008 {published data only}
- Jansi LB. Effect of oil massage on changes in weight and neurobehavioural response of low birth weight babies. The Nursing Journal of India 2008;99(11):256-8. [PMID: ] [PubMed] [Google Scholar]
Mathai 2001 {published data only}
- Mathai S, Fernandez A, Mondkar J, Kanbur W. Effects of tactile-kinesthetic stimulation in preterms: a controlled trial. Indian Pediatrics 2001;38(10):1091-8. [PMID: ] [PubMed] [Google Scholar]
Mendes 2008 {published data only}
- Mendes EW, Procianoy RS. Massage therapy reduces hospital stay and occurrence of late-onset sepsis in very preterm neonates. Journal of Perinatology 2008;28(12):815-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Montaseri 2020 {published data only}
- Montaseri S, Barati R, Edraki M, Hemmati F. The effects of massage therapy with or without physical exercises on the weight of premature infants admitted to the neonatal intensive care unit: a randomized clinical trial. Shiraz E-medical Journal 2020;21(2):e91033. [Google Scholar]
Rutter 1981 {published data only}
- Rutter N, Hull D. Reduction of skin water loss in the newborn. I. Effect of applying topical agents. Archives of Disease in Childhood 1981;56(9):669-72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith SL, Lux R, Haley S, Slater H, Beachy J, Moyer-Mileur LJ. The effect of massage on heart rate variability in preterm infants. Journal of Perinatology 2013;33(1):59-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solanki 2005a {published data only}
- Solanki K, Matnani M, Kale M, Joshi K, Bavdekar A, Bhave S, et al. Transcutaneous absorption of topically massaged oil in neonates. Indian Pediatrics 2005;42(10):998-1005. [PMID: ] [PubMed] [Google Scholar]
Taheri 2018 {published data only}
- Taheri PA, Goudarzi Z, Shariat M, Nariman S, Matin EN. The effect of a short course of moderate pressure sunflower oil massage on the weight gain velocity and length of NICU stay in preterm infants. Infant Behavior and Development 2018;50:22-27. [DOI] [PubMed] [Google Scholar]
Wananukul 2001 {published data only}
- Wananukul S, Praisuwanna P, Kescorncam K. Effects of clear topical ointment on transepidermal water loss in jaundiced preterm infants receiving phototherapy. Journal of the Medical Association of Thailand 2001;84(6):837-41. [PMID: ] [PubMed] [Google Scholar]
Wananukul 2002 {published data only}
- Wananukul S, Praisuwanna P. Clear topical ointment decreases transepidermal water loss in jaundiced preterm infants receiving phototherapy. Journal of the Medical Association of Thailand 2002;85(1):102-6. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hu 2014 {published data only}
- Hu X, Zhang Y. Effect of topically applied sunflower seed oil in preterm infants. Pediatric Critical Care Medicine 2014;15(4 Suppl 1):144-5. [Google Scholar]
Maamouri 2018 {published data only}
- Maamouri G, Abdolhossein Attar M, Rakhshandeh H, Lotfi M, Zakerihamidi M, Boskabadi H. Effect of topical sunflower oil in preventing nosocomial infections in premature infants. The Iranian Journal of Obstetrics, Gynecology and Infertility 2018;21(9):10-17. [Google Scholar]
Nangia 2015 {published data only}
- Nangia S, Paul VK, Deorari AK, Sreenivas V, Agarwal R, Chawla D. Topical oil application and trans-epidermal water loss in preterm very low birth weight infants- a randomized trial. Journal of Tropical Pediatrics 2015;Sep 3:[Epub ahead of print]. [PMID: ] [DOI] [PubMed] [Google Scholar]
Saeidi 2014 {published data only}
- Saeedi R, Gholami M, Dinparvar S, Kabirian M. Transcutaneous feeding: the effect of massage with coconut oil on weight gaining in preterm newborns. Iranian Red Cresent Medical Journal 2011;13:666-9. [Google Scholar]
- Saeidi R, Ghorbani Z, Moghaddam AS. The effect of massage with medium-chain triglyceride oil on weight gain in premature neonates. Acta Medica Iranica 2014;53:134-8. [PubMed] [Google Scholar]
Summers 2019 {published data only}
- Summers A, Visscher MO, Khatry SK, Sherchand JB, LeClerq SC, Katz J, et al. Impact of sunflower seed oil versus mustard seed oil on skin barrier function in newborns: a community-based, cluster-randomized trial. BMC Pediatrics 2019;19(1):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Kumar 2020 {published data only}
- Kumar V, Kumar A, Mishra S, Ashraf S, Singh S, Kan P, et al. Impact of emollient therapy with sunflower seed oil on survival of newborn infants in Uttar Pradesh, India: a community-based, cluster randomised, open-label controlled trial. ISRCTN Registry 2020;https://doi.org/10.1186/ISRCTN38965585.
Additional references
Adams‐Chapman 2006
- Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. The Journal of Pediatrics 2012;160:49-53.e1. [PMID: ] [DOI] [PubMed] [Google Scholar]
BNF for Children 2020
- Paediatric Formulary Committee. BNF for Children. London, UK: BMJ Group, Pharmaceutical Press, and RCPCH Publications, 2020. [Google Scholar]
Camacho‐Gonzalez 2013
- Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatric Clinics of North America 2013;60(2):367-89. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cartlidge 2000
- Cartlidge P. The epidermal barrier. Seminars in Neonatology 2000;5(4):273-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Darmstadt 2002a
- Darmstadt GL, Mao-Qiang M, Chi E, Saha SK, Ziboh VA, Black RE, et al. Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatrica 2002;91(5):546-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Darmstadt 2002b
- Darmstadt GL, Saha SK. Traditional practice of oil massage of neonates in Bangladesh. Journal of Health, Population, and Nutrition 2002;20(2):184-8. [PMID: ] [PubMed] [Google Scholar]
Dyer 2013
- Dyer JA. Newborn skin care. Seminars in Perinatology 2013;37(1):3-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Evans 1986
- Evans NJ, Rutter N. Development of the epidermis in the newborn. Biology of the Neonate 1986;49(2):74-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gordon 2006
- Gordon A, Isaacs D. Late onset neonatal Gram-negative bacillary infection in Australia and New Zealand: 1992-2002. The Pediatric Infectious Disease Journal 2006;25(1):25-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 8 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harpin 1983
- Harpin VA, Rutter N. Barrier properties of the newborn infant's skin. Journal of Pediatrics 1983;102(3):419-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [DOI] [PubMed] [Google Scholar]
Isaacs 2003
- Isaacs D, Australian Study Group for Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(2):F89-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isaacs 2004
- Isaacs D, Fraser S, Hogg G, Li HY. Staphylococcus aureus infections in Australasian neonatal nurseries. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89:F331-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelleher 2021
- Kelleher MM, Cro S, Cornelius V, Lodrup Carlsen KC, Skjerven HO, Rehbinder EM, et al. Skin care interventions in infants for preventing eczema and food allergy. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013534. [DOI: 10.1002/14651858.CD013534.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2017
- Khan AM, Morris SK, Bhutta ZA. Neonatal and perinatal infections. Pediatric Clinics 2017;64(4):785-98. [DOI] [PubMed] [Google Scholar]
Lee 1993
- Lee EJ, Gibson RA, Simmer K. Transcutaneous application of oil and prevention of essential fatty acid deficiency in preterm infants. Archives of Disease in Childhood 1993;68(1 Spec No):27-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchini 2002
- Marchini G, Lindow S, Brismar H, Stabi B, Berggren V, Ulfgren AK, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. The British Journal of Dermatology 2002;147(6):1127-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mullany 2005
- Mullany LC, Darmstadt GL, Khatry SK, Tielsch JM. Traditional massage of newborns in Nepal: implications for trials of improved practice. Journal of Tropical Paediatrics 2005;51(2):82-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ness 2013
- Ness MJ, Davis DM, Carey WA. Neonatal skin care: a concise review. International Journal of Dermatology 2013;52(1):14-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ovelman 2020
- Ovelman C, Eckert C, Friesen C. Validating Cochrane Neonatal’s standard search databases: is it okay to stop searching Embase? In: Advances in Evidence Synthesis: special issue. Cochrane Database of Systematic Reviews 2020;(9(Suppl 1): 320). [DOI: 10.1002/14651858.CD202001] [DOI]
Pickens 2000
- Pickens WL, Warner RR, Boissy YL, Boissy RE, Hoath SB. Characterization of vernix caseosa: water content, morphology, and elemental analysis. Journal of Investigative Dermatology 2000;115(5):875-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pupala 2019
- Pupala SS, Rao SD, Strubk T, Patole S. Topical application of coconut oil to the skin of preterm infants: a systematic review. European Journal of Pediatrics 2019;178(9):1317-24. [DOI: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre: The Cochrane Collaboration, 2020.
Rutter 1988
- Rutter N. The immature skin. British Medical Bulletin 1988;44(4):957-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rutter 2000
- Rutter N. Clinical consequences of an immature barrier. Seminars in Neonatology 2000;5(4):281-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salam 2013
- Salam RA, Das JK, Darmstadt GL, Bhutta ZA. Emollient therapy for preterm newborn infants--evidence from the developing world. BMC Public Health 2013;13(Suppl 3):S31. [DOI: 10.1186/1471-2458-13-S3-S31] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samanta 2011
- Samanta S, Farrer K, Breathnach A, Heath PT. Risk factors for late onset gram-negative infections: a case-control study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F15-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors, GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Available from www.guidelinedevelopment.org/handbook (accessed prior to 5 May 2021).
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2004
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292(19):2357-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vergnano 2011
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F9-F14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2005
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet 2005;365(9465):1175-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cleminson 2016
- Cleminson J, McGuire W. Topical emollient for preventing infection in preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No: CD001150. [DOI: 10.1002/14651858.CD001150.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conner 2008
- Conner JM, Soll R, Edwards WH. Topical ointment for preventing infection in preterm infants. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD001150. [DOI: 10.1002/14651858.CD001150.pub2] [DOI] [PubMed] [Google Scholar]
Edwards 2001
- Edwards WH, Conner JM, Soll RF et al. The effect of Aquaphor Original Emollient Ointment on nosocomial sepsis rates and skin integrity in infants of birth weight 501 to 1000 grams. Pediatric Research 2001;49:388A. [DOI] [PubMed] [Google Scholar]
Soll 1998
- Soll RF, Edwards WH. Emollient ointment for preventing infection in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 3. Art. No: CD001150. [DOI: 10.1002/14651858.CD001150] [DOI] [PubMed] [Google Scholar]


