Fig. 4. Structure of the bacteriophage phi29 dsDNA packaging motor stalled during packaging.
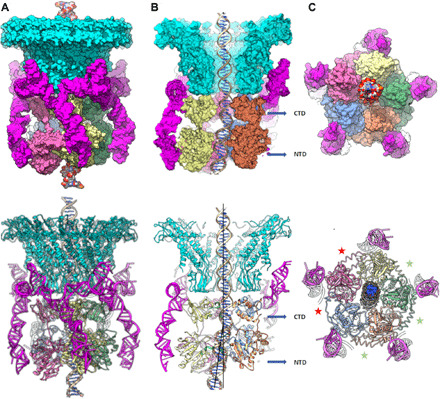
Structure of the packaging motor rendered as molecular surfaces (top panels) and ribbon diagrams (bottom panels) shown from the following: (A) side view; (B) cutaway side view to visualize the DNA in the central channel; and (C) an end-on view, looking from below the motor. The portal and pRNA are colored cyan and magenta, respectively, and the five ATPase subunits are labeled S1 to S5 and colored blue (S1), orange (S2), green (S3), yellow (S4), and pink (S5). Approximate levels of the CTD and NTD are indicated by blue arrows in the panel. Deviations from the rotational component of helical symmetry are shown indicating loose and tight interfaces by red and green asterisks, respectively, in (C); the loose interfaces are on either side of the lowest subunit in the ATPase helix and correspond to the two active sites where there is no clear density for ATP. Two black lines in the bottom half of (B) are drawn approximately coincident with the helical axis of the DNA before and after the kink that occurs between the CTD and NTD; the ~12.5° angle between the lines is indicated.
