Fig. 3. N-glycosylation of integrin α5β1 bridges interface to FN.
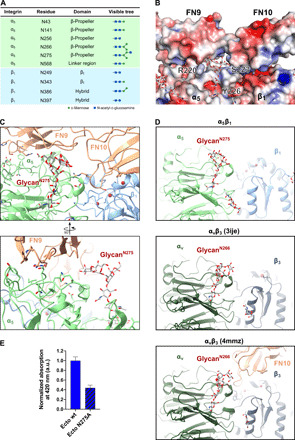
(A) Table listing the N-glycosylation sites and the types of glycan trees as determined in our structure. (B) Electrostatic potential map of the FN-integrin interface and the N275-glycan. (C) Two views of the N275-glycan on α5, which is part of the extensive α5β1-FN interface. The N275-glycan contacts with FN9. (D) Comparison of glycan position between integrin α5β1 and integrin αvβ3 [PDB ID: 3ije (43)] and αvβ3 in complex with FN10 [PDB ID: 4mmz (42)]. The N275-glycan (α5β1) is a conserved glycosylation site among FN-recognizing integrins such as αvβ3. (E) Solid-phase binding assay to measure the binding affinity of integrin α5(N275A)β1 ectodomain mutant in comparison to wild-type ectodomain. Both wild-type and N275A ectodomain mutant were unclasped and binding to FN7-10 wild type evaluated in the presence of Mn2+. Bars represent means + SD of three measurements. Unspecific binding of integrin to bovine serum albumin (BSA)–coated control wells was subtracted from the total absorbance values. Normalized binding is expressed as the ratio of mutant absorbance relative to wild-type absorbance (ratio = 1 represents wild-type binding).
