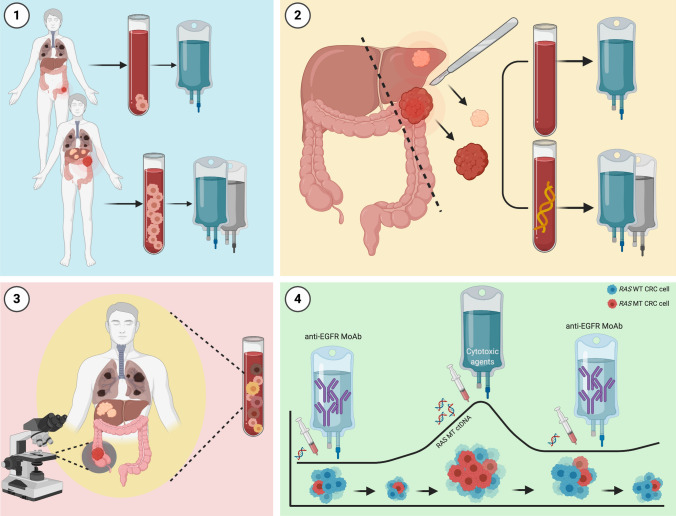
Fig. 3.
Overview of potential clinical applicability for circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in the continuum of care for patients with metastatic colorectal cancer (mCRC). Circulating tumor cells and ctDNA yield potential clinical applicability in treatment guidance for patients with mCRC: (1) the CTC count may guide treatment selection, with regard to therapy intensification and de-escalation; (2) after surgical resection for oligometastatic colorectal cancer (CRC), post-surgical treatment strategies may be modulated according to minimal residual disease (MRD), as determined by ctDNA levels in peripheral blood; (3) ctDNA may refine molecular profiling in mCRC by capturing tumor molecular heterogeneity, which is otherwise unachievable by single-tissue biopsy exploring just one site of the disease; and (4) ctDNA allows clonal tracking and real-time monitoring of emerging resistance, guiding treatment selection and rechallenge with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAb). MT mutant. Created with BioRender.com