Figure 5.
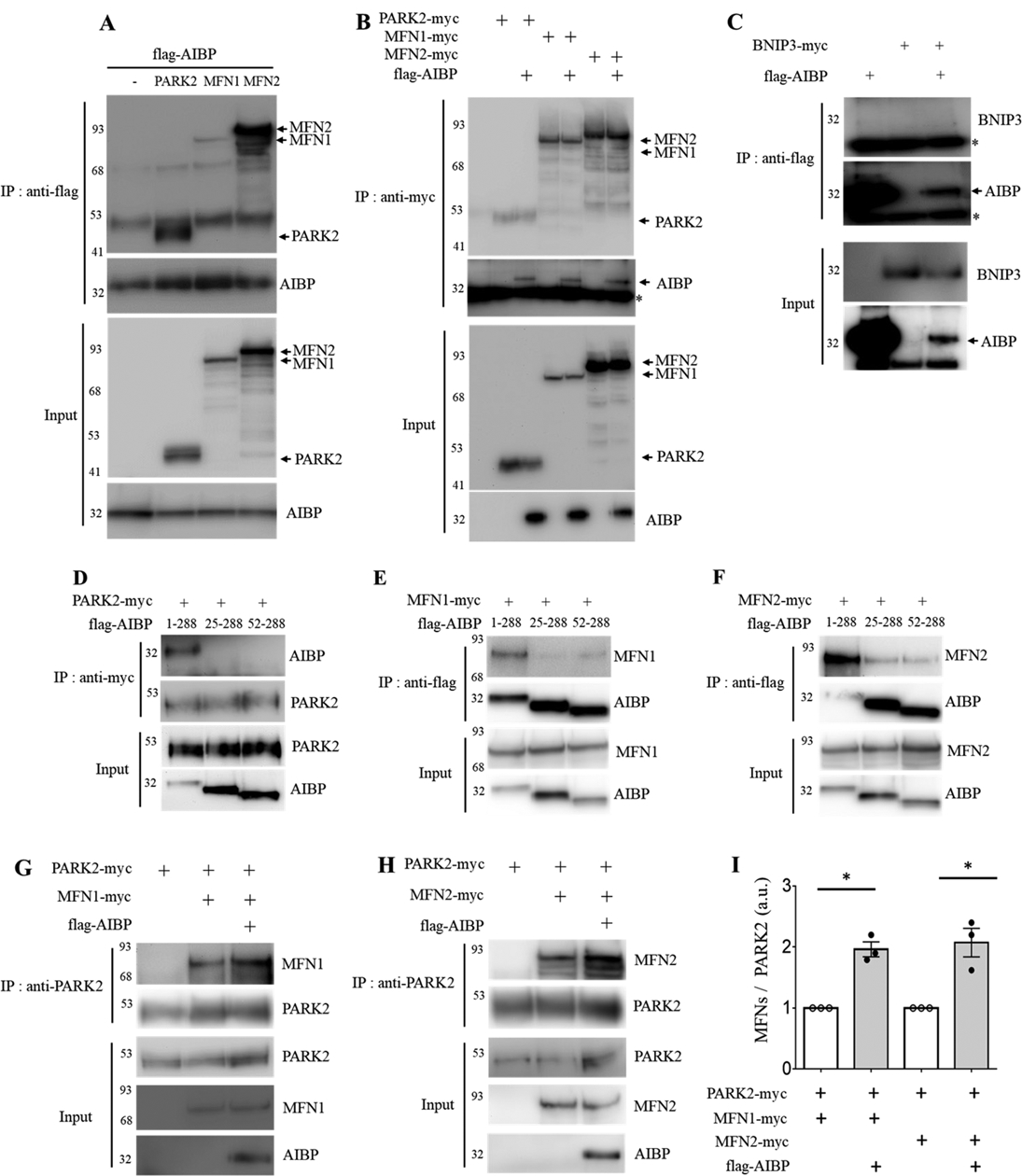
AIBP associates with PARK2, MFN1 and MFN2 and enhances PARK2-MFN1/2 interactions. (A-B) HEK 293 cells were co-transfected with flag-tagged AIBP (1–289 aa) and PARK2-myc, MFN1-myc, or MFN2-myc. (A) AIBP-driven immunoprecipitation with an anti-flag antibody. (B) Immunoprecipitation driven by PARK2, MFN1, or MFN2 with an anti-myc antibody. (C) AIBP does not bind to BNIP3. HEK 293 cells were co-transfected with flag-tagged AIBP and BNIP3-myc. Cell lysates were immunoprecipitated with an anti-flag antibody. *, IgG light chain. (D-F) HEK293 cells were co-transfected with flag-tagged AIBPs (1–289 aa, 25–289 aa, or 52–289 aa) together with (D) PARK2-myc, (E) MFN1-myc, or (F) MFN2-myc. (D) PARK2 and (E and F) AIBPs were immunoprecipitated with an anti-myc or anti-flag antibody, respectively. (G-I) HEK 293 cells were co-transfected with flag-tagged AIBP, PARK2-myc, and MFN1-myc or MFN2-myc. Cell lysates were immunoprecipitated with an anti-PARK2 antibody, and the bound (G) MFN1 and (H) MFN2 were detected by immunoblotting with an anti-myc antibody. (I) Band intensities were measured and MFN/PARK2 ratios calculated. Because of variation in independent transient transfection/pull-down/blot experiments conducted on separate days, experimental samples (with AIBP) were normalized to the control samples in which AIBP was not added. With control samples (no AIBP) set as 1, one-sample t-test was conducted to test the hypothesis whether the experimental group (with AIBP) was different from 1, i.e., whether AIBP significantly increased MFN1 and MFN2 binding to PARK2 compared to no-AIBP controls. Mean±SEM; N=3–4. *, p<0.05; **, p<0.005; ***, p<0.0005; ****, p<0.0001.
