Figure 6.
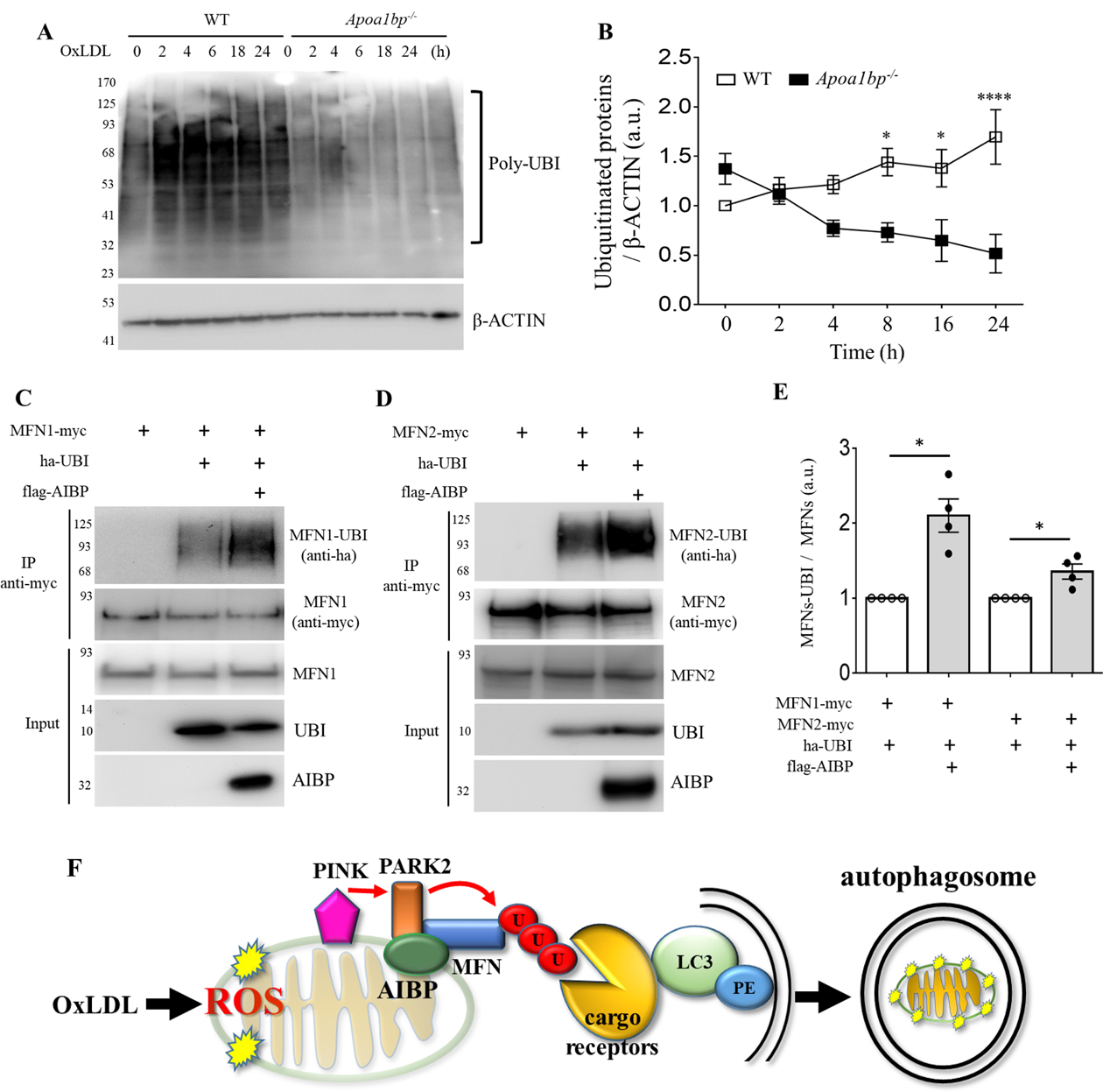
AIBP regulates the ubiquitination pathway. (A and B) BMDM isolated from WT and Apoa1bp−/− mice were incubated with PBS or 25 μg/ml OxLDL for indicated time. Cells were harvested and immunoblotted with anti-UBI and β-ACTIN antibodies. The band intensities were quantified. (C and D) HEK 293 cells were co-transfected with flag-tagged AIBP, ha-ubiquitin, and MFN1-myc or MFN2-myc. Cell lysates were immunoprecipitated with an anti-myc antibody, and ubiquitination was detected by immunoblotting with an anti-ha antibody. (E) Band intensities were measured and MFN-UBI/MFN ratios calculated. Because of variation in independent transient transfection/pull-down/blot experiments conducted on separate days, experimental samples (with AIBP) were normalized to the control samples in which AIBP was not added. With control samples (no AIBP) set as 1, one-sample t-test was conducted to test the hypothesis whether the experimental group (with AIBP) was different from 1, i.e., whether AIBP significantly increased MFN1 and MFN2 ubiquitination compared to no-AIBP controls. Mean±SEM; N=3–4. *, p<0.05; **, p<0.005; ***, p<0.0005; ****, p<0.0001. (F) Schematic overview of the regulation of mitophagy by AIBP. Intracellular AIBP associates with mitochondrial proteins Parkin and MFN1/MFN2 and upregulates ubiquitination of MFN1 and MFN2; in turn ubiquitinated MFN are recognized by cargo receptors interacting with lipidated LC3 anchored in the membrane to form an autophagosome. In the context of atherosclerosis, this regulation is particularly important in removal of damaged mitochondria in macrophages exposed to atherogenic OxLDL.
