Figure 2:
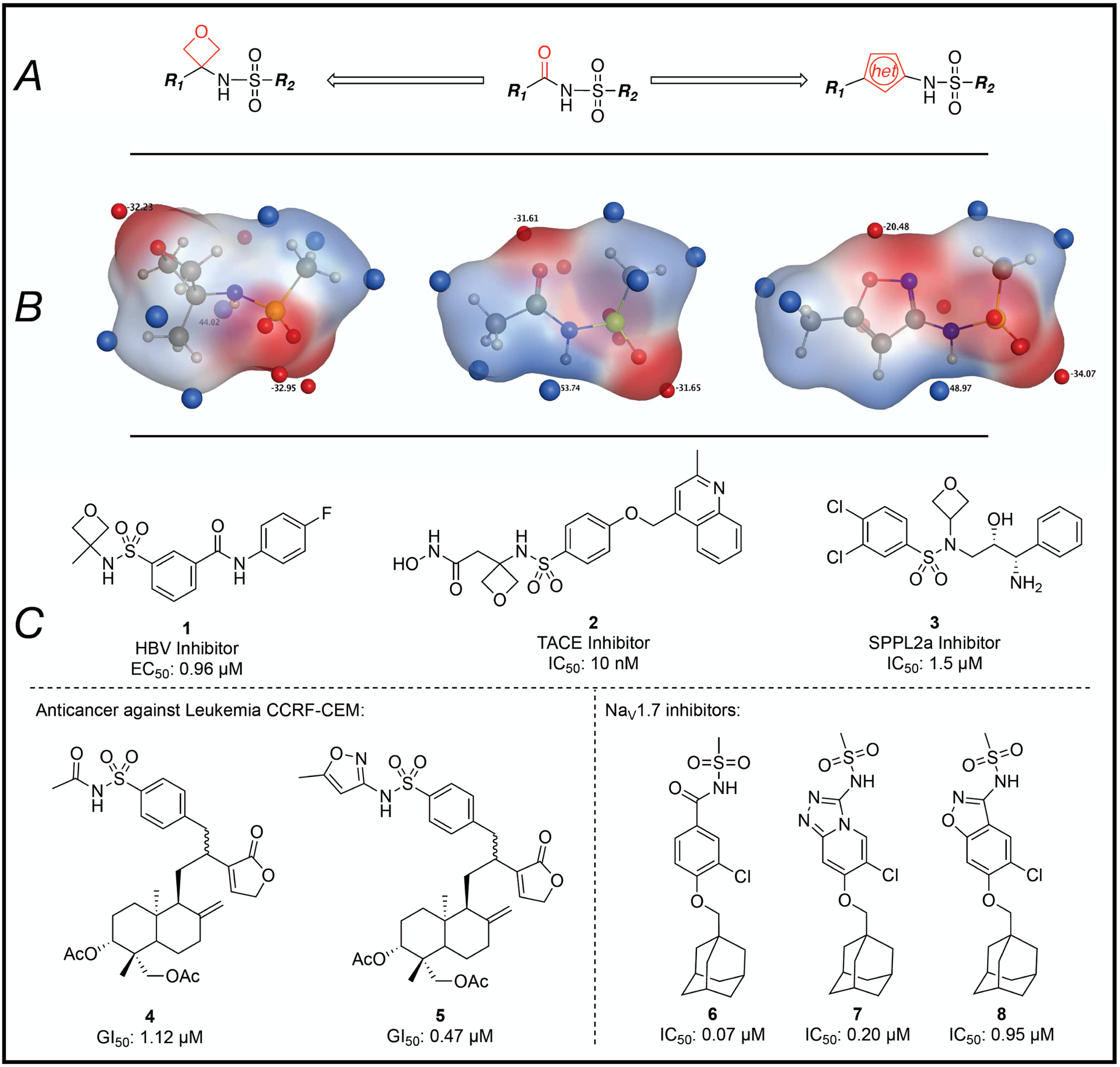
(A) General outline of N-acylsulfonamide bioisosteres that are based on replacement of the carbonyl moiety with either an oxetane or a 5-membered ring heteroaromatic ring. (B) comparison of Gaussian-optimized geometry and electrostatic potential maps of the N-acylsulfonamide moiety (center) and the corresponding oxetane (left) and isoxazole (right) derivatives. The areas colored in red and blue represent respectively negative and positive regions of the electrostatic potential; the corresponding surface minima and maxima are indicated as red and blue spheres. (C) Representative literature examples,37–39 including match paired comparisons, 4 and 5, as well as 6–8.35, 36
