Scheme 1.
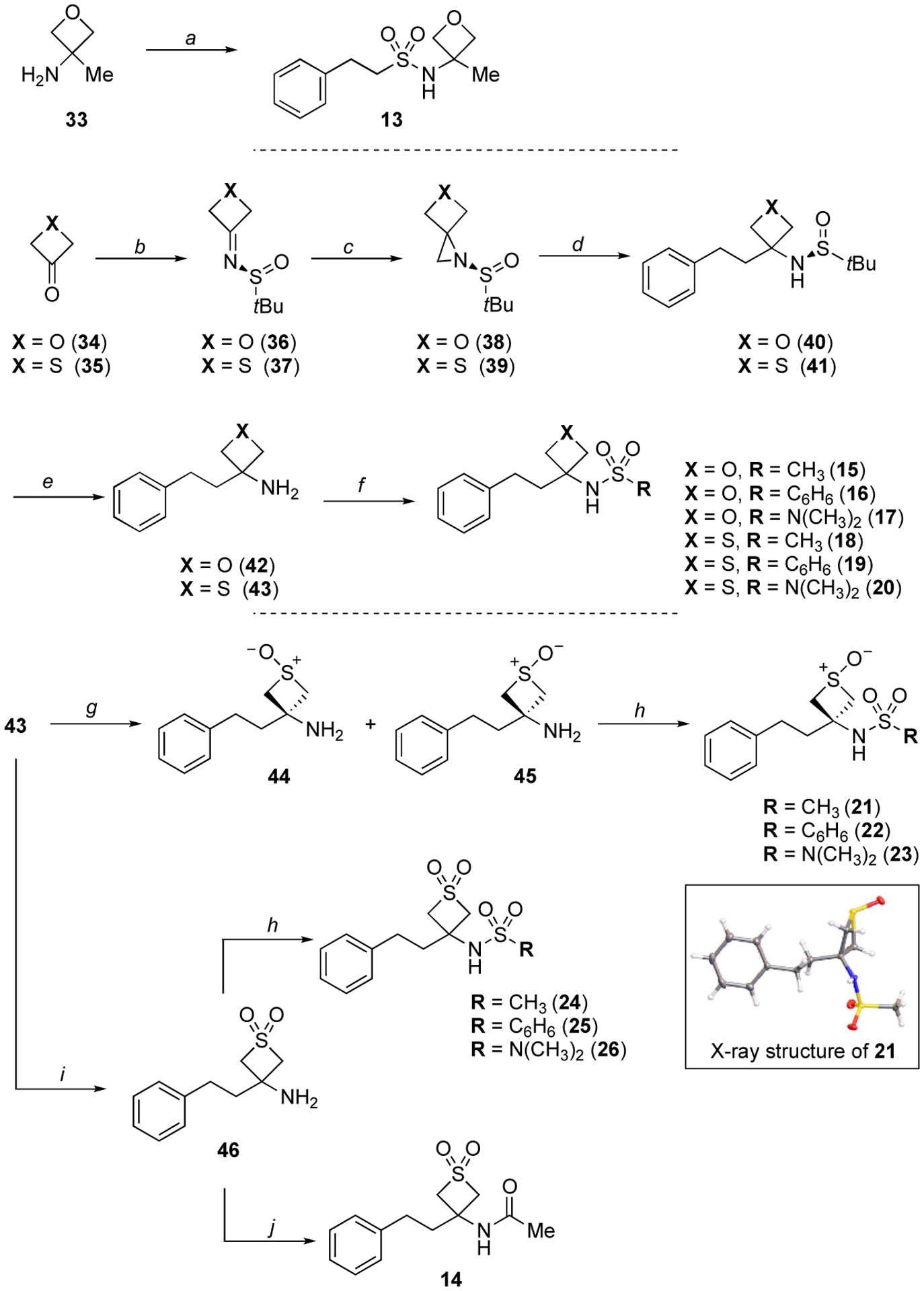
Reagents and conditions: (a) Et3N, 2-phenylethane-1-sulfonyl chloride, CH2Cl2, 0 °C to rt, 16 h, (34%); (b) (S)-(−)-2-methyl-2-propanesulfinamide, Ti(iPrO)4, CH2Cl2, reflux, 16 h, (77%); (c) TMSOI, NaH, DMSO, rt, 2 h, (39–49%); (d) benzylmagnesium chloride, CuI, THF, −30 to 0 °C, 1 h, (48–90%); (e) HCl, CH3OH, 0 °C to rt, 16 h, (62%); (f) Et3N, appropriate sulfonyl chloride or sulfamoyl chloride, CH2Cl2, 0 °C to rt, 16 h, (24–68%); (g) m-CPBA, CH2Cl2, −78 °C, 2 h, (70%); (h) Et3N, appropriate sulfonyl or sulfamoyl chloride, CH2Cl2, 0 °C to rt, 16 h, (15–78%); (i) oxone, acetone/H2O (1:1), 0 °C to rt, 16 h, (47%); (g) acetic anhydride, AgOTf, CH2Cl2, 0 °C to rt, 2 h, (67%).
