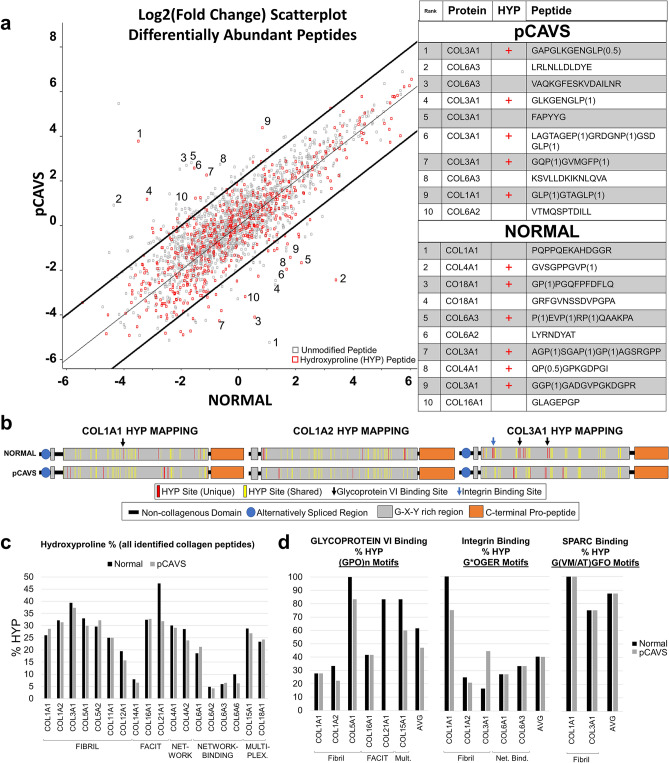
Figure 4.
Pediatric CAVS valves have reduced HYP, including in collagen-ECM binding sites. (A) A log2(fold change) scatterplot of pCAVS versus Normal peptides. Red squares are hydroxyproline containing peptides, gray squares are unmodified peptides. Bold lines indicate X + 2 and X − 2. Top 10 collagen peptides are annotated with corresponding sequence and protein shown (A, right). P(X) where X is between 0–1, indicated HYP probability at that site. Significantly regulated collagen subtypes differ between pCAVS and normal, with non-fibril types (multiplexin, FACIT) differentially regulated in normal. (B) Representative hydroxyproline site mapping for collagen 1A1, collagen 1A2, and collagen 3A1, shown for Normal and pCAVS patients (DB27 and DB16, respectively). Normal sample contained more HYP sites than age matched controls. Unique HYP sites in Normal (top, red) are found in glycoprotein vi and integrin binding motifs (black and blue arrows, respectively). HYP is required in these motifs for binding. (C) Quantification of hydroxyproline state of proteins in all peptides identified in normal (black) or pCAVS (gray). (D) Hydroxyproline percent in peptides containing glycoprotein vi, integrin, or SPARC binding motifs. Loss of HYP may indicate lack of collagen-ECM binding. FACIT: Fibril-associated collagen with interrupted triple helices; Net.: Network Collagen; Net. Bind: Network Binding Collagen; Mult.: Multiplexin (Multiple triple-helix domains with interruptions). Normal n = 4, pCAVS n = 11. MaxQuant and Perseus v.1.6.3.3. was used for this analysis (https://maxquant.net/maxquant/).