Abstract
Objective
To identify factors associated with job satisfaction and retention, we surveyed a large cohort of clinical research coordinators (CRCs). In recent years, the clinical research coordinator has changed from a semi-permanent role to one that has a high turnover rate. The CRCs are integral to clinical research and instability in this role can cause patient stress and increase the burden on clinical teams through unnecessary delegation of resources toward hiring and retraining new talent. The cultural shift toward CRCs as a temporary position may be driven by the perspective that the role positions an individual for other health care careers, but understanding what influences low retention rates are necessary.
Methods
A survey containing 13 multiple choice or open-ended and 32 Likert scale questions was distributed to previous and current CRCs using REDCap. The questionnaires were self-administered and completed over a 12-month period between October 11, 2017, and September 16, 2018.
Results
A total of 85 CRCs completed the study. From the 32 potential predictors of retention, we investigated 9 significant predictors: salary, work setting, understanding the role, level of CRC, understanding protocol development, actively engaged principal investigator (PI), having a collaborative role with PI, feeling respected by PI, and having a close relationship with PI. Adequate salary, greater respect, collaboration, and engagement from the PI were significantly associated with higher retention. Surprisingly, greater workload and lack of opportunity for professional growth were not associated with retention.
Conclusion
The CRCs who feel respected and engaged by the PI and are adequately compensated are more likely to have higher job satisfaction and retention.
Abbreviations and Acronyms: CRC, clinical research coordinator; OR, odds ratio; PI, principal investigator
Clinical research studies are conducted by a multidisciplinary research team including but not limited to clinical staff, regulatory staff, and administrative personnel. The coordination among such a comprehensive team is often led by a clinical research coordinator (CRC), whose daily responsibilities vary significantly but ultimately serve as the liaison between study sponsor and patients and clinical, regulatory, and administrative staff. The CRCs are integral for the success of clinical research due to their wide range in responsibilities. Clinical research coordinators are typically composed of clinical research nurses, clinical trial nurses, research nurse coordinators, and study coordinators.1 The main tasks that CRCs conduct for multicenter trials include recruitment of patients into clinical trials (screening, enrollment, consent, and randomization), conducting study visits (collecting biofluids, monitoring compliance, and documenting adverse events), maintaining study regulatory documents, reporting adverse events to the appropriate regulatory authorities (eg, institutional review board, sponsor, US Food and Drug Administration, and National Institutes of Health, conducting research in accordance with Good Clinical Practice guidelines, managing study budgets, maintenance of electronic study records, and general liaison between study sponsor and site principal investigator (PI).
Hiring a CRC represents a significant investment in time and training to properly carry out job responsibilities. Turnover in this role is costly; these costs are related to salary, effort of the employee and trainee, time for education and development, marketing and recruitment, loss of productivity during orientation and training, and emotional costs of turnover on current staff.2 Because of this investment, it is advantageous to retain individuals in this position for extended periods, positioning this role as a long-term career. Currently the CRC role has become a temporary position in which coordinators seek to gain clinical experience before medical school, nursing positions, or other graduate programs.
With the increase in regulatory oversight among clinical trials, the demands and expectations of CRCs have increased and require additional skills, training, and medical knowledge. Depending on experience and interest, CRCs may actively participate in additional investigator-initiated projects, manuscript preparation, and conference presentations. The expansion of the CRC role includes additional responsibilities and increased workload for individuals, which adversely affect their job satisfaction and ultimately duration in the position. A study by Anderson and Milkovich found that job satisfaction was a significant predictor of retention with professional, managerial, and technical employees.3 Similarly, Gullatte and Jirasakhiran2 found that lack of job satisfaction was the most cited reason for staff turnover.
Given the important role of CRCs on the success of clinical research and the necessary investment of resources for their success, it is advantageous to identify factors associated with job satisfaction and increased retention. There is limited literature evaluating these factors and the existing studies are less relevant considering the rapid pace at which the structure of clinical research evolves. This study sought to identify specific predictors of retention from 32 different potential factors.
Methods
A 45-question online survey was developed by the clinical research team at Vanderbilt University Medical Center and administered over a 12-month period between October 11, 2017, and September 16, 2018. Questions were based on anecdotal reports from CRCs, clinicians and clinical research staff within the clinic, clinicians and research staff at the University of Rochester, and research administrators within the Huntington Study Group. The 45-question self-administered questionnaire used a combination of multiple choice and open-ended (questions 1-13) and 5-point Likert scale (questions 14-45) questions ranging from strongly disagree (0) to strongly agree (5), outlined in Tables 1 and 2. The questions addressed research setting, duration in position, prior experiences, relationships with study participants and research staff, compensation, professional support, additional training opportunities, and opportunity for career advancement. Information for participant retention was recorded using an ordinal response category with the following duration categories: 0 to 1, 1 to 5, 6 to 10, 11 to 16, and more than 16 years.
Table 1.
Multiple-Choice and Open-Ended Survey Questions
| Question No. | Question | Response |
|---|---|---|
| 1 | Were you a coordinator before your current position? | Yes/no |
| 2 | Do you currently hold, or have you previously held the position of a clinical trial coordinator? | Current/previous |
| 3 | What level research position are you (CRC, assistant, or associate)? | 1, 2, 3, 4 |
| 4 | Are you on a sponsor-initiated trial or investigator-initiated trial? | Sponsor/investigator |
| 5 | How long have you been a coordinator? | Open ended |
| 6 | Which phrase is more applicable to why you became a coordinator? | Transition from clinical care, considered research a career, not happy in previous work, gain more research, other |
| 7 | Did you see this position as temporary or a career? | Temporary/career |
| 8 | From the following, what would interest you in addition to your current position’s duties (only choose 1)? | Social work, basic science, administrative tasks, clinical care, other |
| 9 | Do you work in an academic, hospital, or private research setting? | Academic, hospital, private |
| 10 | What kind of training did you receive when you first started? | Open ended |
| 11 | Was your training specific to a disease state? | Yes/no |
| 12 | Currently do you participate in noncoordinating tasks? | Yes/no |
| 13 | How many studies do you manage? | Open ended |
CRC = clinical research coordinator.
Table 2.
Five-Point Likert Scale Survey Questions
| No. | Question | Likert Scale Responses |
||||
|---|---|---|---|---|---|---|
| Strongly Disagree (0) | Disagree (1) | Neutral (2) | Agree (3) | Strongly Agree (4) | ||
| 1 | Before starting, I understood the role of a clinical research coordinator. | |||||
| 2 | A clinical research coordinator has much autonomy. | |||||
| 3 | I believe that my training for the role of a clinical research coordinator was adequate. | |||||
| 4 | Clinical research coordinators have opportunities for professional growth. | |||||
| 5 | My position is well respected in the health care world. | |||||
| 6 | I believe I have a good salary. | |||||
| 7 | I have a collaborative role with my PI. | |||||
| 8 | I have a close working-relationship with my PI. | |||||
| 9 | I feel respected by my PI. | |||||
| 10 | My PI is actively engaged in my research. | |||||
| 11 | My work is appreciated by others. | |||||
| 12 | The PI I work with is invested in letting me explore other research opportunities. | |||||
| 13 | I am interested in the disease I study. | |||||
| 14 | My work load is not overly burdensome. | |||||
| 15 | I work in a healthy environment. | |||||
| 16 | I have many opportunities for networking. | |||||
| 17 | The patient population I study is easy to work with. | |||||
| 18 | I get along with my coworkers. | |||||
| 19 | Technology is readily available for me to be successful. | |||||
| 20 | Patient interactions are rewarding. | |||||
| 21 | I have enough time to get all my work done. | |||||
| 22 | I am able to attain help whenever I need it. | |||||
| 23 | I fully understand the nuances of clinical protocol. | |||||
| 24 | I understand aspects of protocol development outside of day-to-day study visits. | |||||
| 25 | The PI provides additional responsibilities outside of a typical coordinator role. | |||||
| 26 | I have opportunities for promotion and professional growth. | |||||
| 27 | My work environment was well structured. | |||||
| 28 | I have opportunities to learn and explore new ideas. | |||||
| 29 | I was able to contribute to publications and conference presentations. | |||||
| 30 | I was offered incentive pay. | |||||
| 31 | Salary increases were directly tied to my productivity. | |||||
| 32 | The institutional structure for salary was clearly defined. | |||||
PI = principal investigator.
The study was administered electronically through the secure web platform REDCap, and participants were identified using the Huntington Study Group list serv and professional contacts. The survey was distributed to 113 participants identified as previous or current CRCs from 32 academic medical centers. We collected participant email address, sex, and work setting but did not collect information related to age, previous work background, level of education, or job title (ie, CRN, clinical research associate, or clinical research nurse).
Statistical Analyses
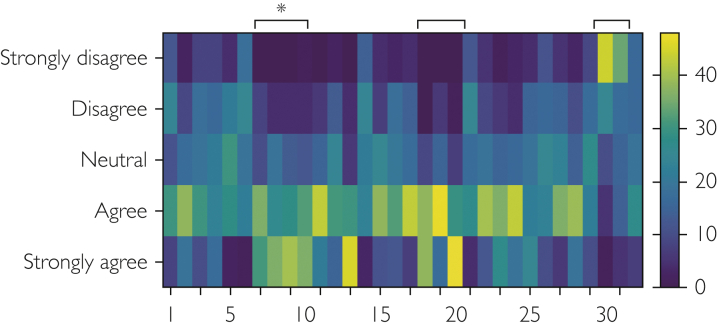
Predictors of retention were identified using logistic regression models with select variables as potential modifiers. Variables were selected based on a principal component analysis bi-plot that identifies groups of predictors with similar responses (Statistical Software R, version 3.5.2). Figure 1 is a visual representation of this bi-plot that identifies the select questions (predictors) that cluster together. This analysis identified 3 clusters of questions containing 9 Likert-scale predictors to be investigated for their role in retention. Single predictor logistic regressions were run on these groups of questions that primarily related to compensation and relationship with the PI. Retention was examined as an ordinal and binary variable (low = 0-5 years and high = 6+ years), yielding no differences in significant predictors between the 2 statistical models and thus only binary comparisons are reported here with the corresponding odds ratio and P value. In an attempt to identify the single most important predictor of retention, we generated a regression model that included all 9 significant predictors of retention. We did not adjust for multiple comparisons. Last, we also examined 8 additional predictors with no clear retention trends but were selected based on anecdotal information. A description of the regression analyses for these variables are outlined in Table 3. All predictors were examined using an ordinal regression with the exception of “work setting,” which was analyzed using a nominal logistic regression as seen in Figure 3(GraphPad Prism, version 8.3, and the Statistical Software R version 3.5.2).
Figure 1.
Response trends for Likert scale questions. Responses for the 32 Likert scale questions identify several clusters of questions with similar response trends. Each vertical column represents a different question with the number of every fifth question identified on the x-axis (1, 5, 10, etc). The number of participant responses from strongly disagree to strongly agree for each box is indicated with different shades of blue to yellow: blue indicates fewer responses and yellow indicates a greater number of responses as estimated with the vertical scale bar. Clusters of questions are indicated with black bars with an asterisk (∗) identifying a cluster of questions related to the role of the principal investigator that were all significant predictors of retention.
Table 3.
Detailed Univariate Regression Analysesa
| Predictor | Estimateb | CI | P |
|---|---|---|---|
| Work setting | 0.253 | 0.08-0.63 | .007 |
| CRC level | 3.75 | 0.02-0.29 | <.001 |
| Good salary | 0.434 | 1.0-2.3 | .043 |
| Understanding role of CRC | 0.608 | 0.41-0.87 | .009 |
| Understood aspects of protocol development | 2.18 | 1.2-4.1 | .01 |
| PI actively engaged | 1.94 | 1.1-3.6 | .02 |
| Collaborative role with PI | 1.77 | 1.0-3.1 | .03 |
| Felt respected by PI | 2.38 | 1.3-4.6 | .006 |
| Close relationship with PI | 3.80 | 1.7-6.0 | <.001 |
| Number of trials | 1.06 | 0.9-1.2 | .453 |
| Professional growth opportunities | 1.17 | 0.82-1.6 | .375 |
| Healthy work environment | 1.0 | 0.64-1.5 | >.999 |
| Rewarding patient interactions | 1.3 | 0.84-3.2 | .167 |
| Having enough time to get their work done | 0.915 | 0.62-1.3 | .646 |
| Publication and conference presentation | 1.47 | 1.0-2.3 | .06 |
| Adequacy of training | 1.27 | 0.87-1.9 | .26 |
CRC = clinical research coordinator; PI= principal investigator.
The β estimate refers to the odds ratio for the corresponding predictor.
Ethical Considerations
The Vanderbilt University Medical Center Institutional Review Board deemed this survey as exempt because the study poses minimal risk to participants. This study meets 45 CFR 46.101 (b) category (2) for Exempt Review; therefore, a consent form was not used. All responses were deidentified by a participant ID number generated by REDCap.
Results
A total of 85 participants completed the survey between October 2017 and September 2018 from the original 113 questionnaires, for a response rate of 75%. Twelve participants were previous CRCs, whereas 73 were current CRCs. There were 9 male and 76 female participants and 7% (n=6) worked exclusively in an academia, 68% (n=60) worked in a hospital, and 25% (n=22) worked in a private setting. The cluster analysis in Figure 1 visually displays similarities in participant responses for Likert scale questions and identifies several clusters of questions with similar response patterns, including a group of 4 questions relating to the role of the PI. Questions within these clusters were the primary focus for factors predicting retention.
Institutional Factors That Affect Retention
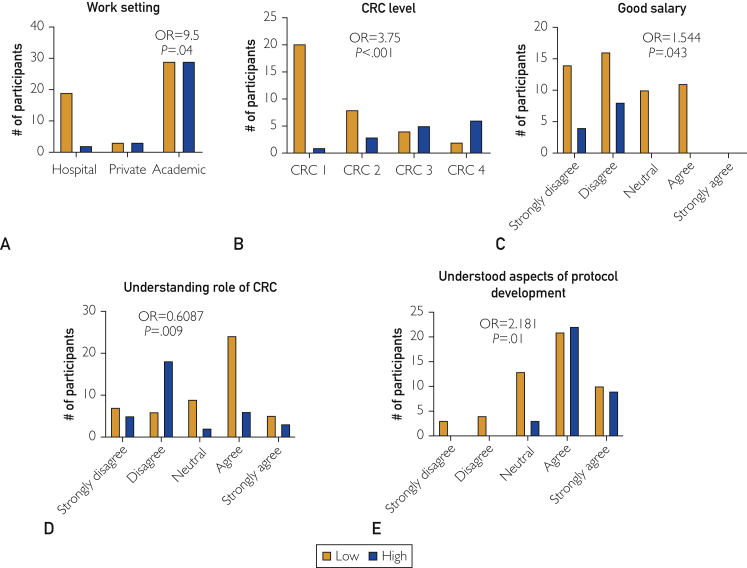
We examined 5 institutional factors as predictors of retention: job setting (P=.04), salary (P=.043), CRC job level (P<.001), understanding job responsibilities (P=.009), and understanding protocol development (P=.01) are all significant predictors of retention (Figure 2). We also examined categorical predictors (CRC level and work setting) based on anecdotal interactions with peers. Participants were 9.5 times more likely to have high retention if they worked in an academic setting compared with a private hospital (P=.04). Participants with increasing satisfaction with their good salary were 1.544 times more likely to have high retention compared with participants less satisfied with their salary (P=.043). Last, respondents who had a better understanding of protocol development were 2.181 times more likely to have high retention (P=.01). For every increase in CRC level, there is a 3.75 increase in the odds of high retention (P<.001). Interestingly, better understanding of the CRC role before starting the position (Figure 2D) was associated with reduced likelihood of high retention (odds ratio, 0.6087; P=.009).
Figure 2.
Five significant predictors of retention. Agreement with: (A) work setting, (B) clinical research coordinator (CRC) level, (C) good salary, (D) understanding role of CRC, and (E) understood aspects of protocol development. Low retention (Low) defined as 0 to 5 years, and high retention (High) defined as 6 or more years. OR = odds ratio.
Role of PI on Retention
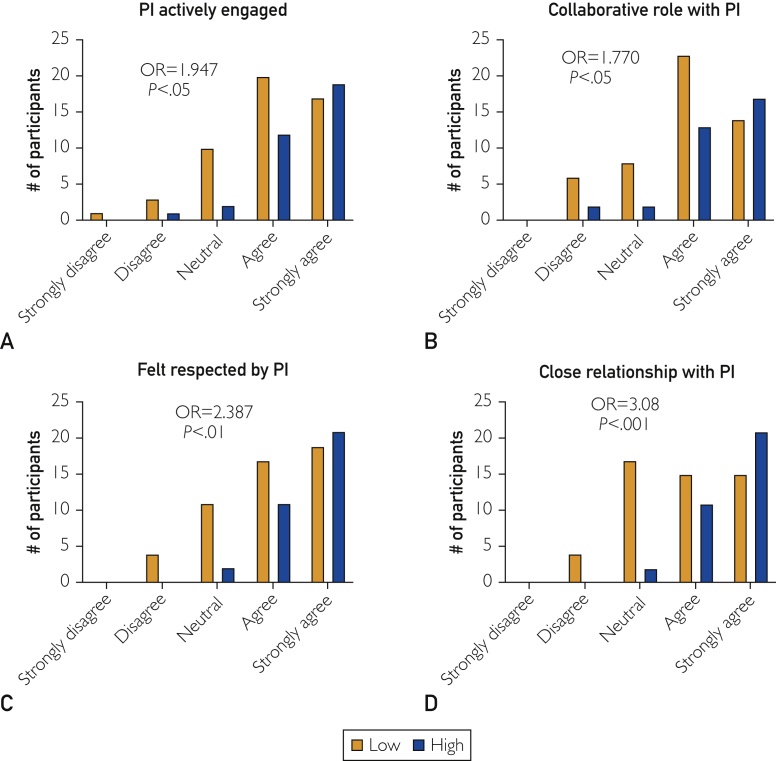
Another major predictor of retention among current and previous CRCs related to the role of the PI. The PI had a significant impact on CRC retention when it relates to being collaborative, engaged, and respectful (Figure 3). The influence of the PI on CRC retention is substantial because there were 4 independent predictors relating to the PI. Participants who believed they had a collaborative role, had a close working relationship, were well-respected, and thought that their PI was actively engaged were 1.770, 3.08, 2.387, and 1.947 times more likely, respectively, to have high retention (P<.05, P<.001, P<.01, and P<.05).
Figure 3.
Four significant predictors of retention related to the role of the principle investigator (PI). Agreement with (A) PI engagement, (B) collaborative role, (C) respect, and (D) a close relationship with the PI was associated with greater odds of higher retention. Low retention (Low) defined as 0 to 5 years, and high retention (High) defined as 6 or more years. OR = odds ratio.
To identify the single most important predictor of retention, we included all 9 significant predictors in a regression model. In this model, the only predictor that retained its significance was having a close working relationship with the PI (data not shown), suggesting that this particular variable best predicts retention.
Factors Unrelated to Retention
The remaining 7 predictors that were not associated with retention were number of trials, professional growth opportunities, healthy work environment, rewarding patient interactions, well-structured work environment, publications and conference presentations, and having enough time to get their work done.
Discussion
Clinical research coordinators are largely invisible participants in clinical research4 but their role is critical. According to the Association of Clinical Research Professionals, the success of clinical trials relies on CRCs, who carry out many of the key clinical, administrative, and regulatory compliance duties.5 A study in 1996 revealed that CRCs performed more than 128 different responsibilities.6 The CRCs usually interact with patients more than the PI for the duration of a trial. This includes study assessments, scheduling, escorting patients around research facilities, being a caregiver, and even being a patient advocate.1 Cooper and Lomax7 state that CRCs make a significant difference in participant recruitment, retention, and study efficiency. The CRCs are not only advocating for the success of the particular study but many times advocate for successful patient treatments. Those roles create a balancing act that the coordinator must face.4 Research coordinators are there to make sure that patients have a good understanding of the protocol, help patients make an informed decision on whether to participate in a trial, the voluntary nature of a clinical trial, and the ability to withdraw from the study at any point without consequences.4 Although the PI is responsible for the overall conduct of the clinical trial, the CRC is responsible for the daily activities.5
Due to the number of roles or duties for which a coordinator is responsible, it is difficult and time-consuming to hire and train new coordinators. Although historically CRCs were considered more long-term career positions, they are more recently viewed as temporary jobs used to provide clinical experience for medical school applications or more competitive clinical research positions. The high turnover also has a detrimental effect on patients because they rely on the coordinator to advocate for them. Although it remains unclear why the CRC is now considered a transient position, 1 potential explanation is the lack of professional identity for these roles, leading to unclear training requirements and job criteria.4 Thus, we sought to better understand factors associated with CRC retention.
We found several predictors of job retention among current and previous CRCs. These predictors relate to job setting, understanding of the position, and salary, as well as another group of predictors related to the role of the PI. Specifically, we found that individuals working in an academic or private research setting had a nearly 10 times greater odds of higher retention (OR=9.5) compared with individuals in a hospital environment. One potential explanation for this association is the well-known discrepancy in salaries across academia, medical centers, and private research institutions, although salary did not significantly alter the relationship between work setting and retention (P=.04).
Clinical research coordinator is often an entry-level position and has salaries corresponding to clinical positions with little or no experience. However, it is clear that the odds of having higher retention is 1.54 times more in those who considered their salary competitive (Figure 2). It then becomes imperative that medical centers and academic institutions conduct ongoing market surveys for proper compensation.2 It is also important for employers to systematically acknowledge and compensate CRCs for increased productivity and job duration. Figure 2 shows that approximately 60% of participants with a low retention group noted a low salary. A study by Gardner et al8 found that pay level was significantly related to both organizational self-esteem and performance. Furthermore, employees who received higher pay felt more highly valued and in return had higher job performance.9 Interestingly, no participant endorsed being compensated based on job productivity. These studies suggest that higher salaries or even financial incentives may promote increased productivity and longer retention among employees.
Relatedly, CRC position was another significant predictor of retention among participants. Position level typically corresponds to an individual's previous experience or reflects job duration. It seems likely that the association between CRC level and retention may also be influenced by salary, promotion success, and support of the institution.
Understanding the role and responsibilities of a CRC position (including protocol development) are additional predictors of retention. Interestingly, the more an individual understands the responsibilities of the CRC position, the less likely they are to have high retention (Figure 2D). This may be a result of the breadth of responsibilities and the increase in administrative burden on the position compared with the hands-on clinical experience most employees seek in this role. It is possible that the relationship between retention and understanding the role of the CRC is affected by an individual’s education level, although for this study we did not specifically gather information regarding respondents' previous education levels or training experience. Interestingly, the survey included a question related to adequacy of training (Table 2, question 3) for their current CRC role, and this was not a significant predictor of retention. Conversely, an individual’s involvement in protocol development results in 2.181 greater odds of higher retention. Participation in protocol development would occur primarily in investigator-initiated trials and a coordinator may feel a greater sense of autonomy and understanding of clinical research.
The second primary cluster of significant retention predictors related to the role of the PI. It is common to see PIs who oversee a large amount of clinical trials, have extensive clinical responsibilities, are committed to expanding investigator-initiated trials, run laboratories, and teach, in addition to travel requirements. Although the National Institutes of Health define the PI as the individual who is primarily responsible for the clinical trial,10 it is clear to all research staff that CRCs more often complete many of the responsibilities formally designated to the PI.11 The demands on a successful PI are extensive and as a result, a CRC may feel a lack of engagement or support from their PI and other research staff. Participants who did not feel engaged with their PI were 90% less likely to have high retention (Figure 3A). A PI who is actively engaged with a sponsor-initiated CRC would be readily available to sign source documents, identify potential study participants, and review laboratory test results and other time-sensitive documents that sponsors may require. A PI who is actively engaged in the research outside of a clinical trial would help with data interpretation and provide opportunities for authorship5 and professional growth.
Additionally, we found that the odds of having higher retention is 77% greater in participants who thought that their PI was collaborative (Figure 3B). According to a recent study, effective managers are identified as those who take time to manage relationships with their employees as well as their bosses.12 It is clear that investing in a professional relationship between CRC and PI involves utilizing each other's strengths and making up for each other's weaknesses.12 Clinical research is a multidisciplinary field that relies heavily on the camaraderie of all participants. As such, the stronger the relationship between team members and the PI, the longer people will want to stay. To this extent, we found that a participant is more than 3.08 times more likely to have high retention when they have a close working relationship with their PI (Figure 3D). Furthermore, in a multiple regression model that includes all 9 significant predictors identified in this study, having a close working relationship with the PI is the single most important predictor of retention. A previous study found that employers who demonstrated a sense of motivation, interest, and concern for their employees' well-being led to higher retention.13
Respect from the PI is the final predictor of retention. One reason for high turnover rates among health care professionals is perhaps due to a lack of recognition and positive feedback from their employers.9 To this end, we found that the odds of having higher retention is 2.387 times more likely than for someone who believed they were not respected by their PI (Figure 3C). According to Sow et al,13 employees have higher retention in organizations in which the employer provides consistent positive feedback and recognition.
Conclusion
Ultimately, we found that although salary and compensation were a significant predictor of retention among this select population of clinical research staff, greater predictors of retention related to the involvement and relationship with the PI. These findings will have significant implications for PIs, who may have limited resources or limited institutional means with which to increase salaries. We show that the investment in time to build genuine relationships with study staff is just as likely to increase long-term retention as better compensation.
Acknowledgements
This work was supported by Huntington Study Group Executive Coordinator Council.
Footnotes
Potential Competing Interests: The authors declare no competing interests.
References
- 1.Fujiwara N., Ochiai R., Shirai Y., et al. Qualitative analysis of clinical research coordinators’ role in phase I cancer clinical trials. Contemp Clin Trials Commun. 2017;8:156–161. doi: 10.1016/j.conctc.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gullatte M.M., Jirasakhiran E.Q. Retention and recruitment: reversing the order. Clin J Oncol Nurs. 2005;9(5):597–604. doi: 10.1188/05.CJON.597-604. [DOI] [PubMed] [Google Scholar]
- 3.Anderson J.C., Milkovich G.T. Propensity to leave: a preliminary examination of March and Simon’s model. Relations Industrielles / Industrial Relations. 1980;35(2):279–294. [Google Scholar]
- 4.Davis A.M., Chandros Hull S., Grady C., Wilfond B.S., Henderson G.E. The invisible hand in clinical research: the study coordinator’s critical role in human subjects protection. J Law Med Ethics. 2002;30(3):411–419. doi: 10.1111/j.1748-720x.2002.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willis C., Bratcher K., Kenworthy-Heinige T., et al. The anatomy of a great clinical research coordinator. Clinical Researcher. 2018. https://acrpnet.org/2018/08/14/the-anatomy-of-a-great-clinical-research-coordinator/ Accessed December 17, 2020.
- 6.Papke A. The ACW national job analysis of the clinical research coordinator. The Monitor. 1996;Winter:45-53.
- 7.Cooper J., Lomax J. The role of the research nurse in clinical trials. Br J Clin Pract. 1989;42:167–168. [PubMed] [Google Scholar]
- 8.Gardner D.G., Van Dyne L., Pierce J.L. The effects of pay level on organization-based self-esteem and performance: a field study. J Occup Organ Psychol. 2004;77(3):307–322. [Google Scholar]
- 9.Chitra K. Role of leaders in employee retention – a pragmatic study with reference to private sector bank employees. Int Res J Bus Manag. 2013 http://irjbm.org/irjbm2013/December/Paper8.pdf [Google Scholar]
- 10.Honari S., Caceres M., Romo M., Gibran N.S., Gamelli R.L. The role of a burn research coordinator: a guide for novice coordinators. J Burn Care Res. 2016;37(2):127–134. doi: 10.1097/BCR.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 11.Haley S.J., Southwick L.E., Parikh N.S., Rivera J., Farrar-Edwards D., Boden-Albala B. Barriers and strategies for recruitment of racial and ethnic minorities: perspectives from neurological clinical research coordinators. J Racial Ethn Health Disparities. 2017;4(6):1225–1236. doi: 10.1007/s40615-016-0332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabarro J.J., Kotter J.P. Managing your boss: a compatible relationship with your superior is essential to being effective in your job. Harv Bus Rev. 1980;58(1):92–100. [PubMed] [Google Scholar]
- 13.Sow M., Ntamon A., Osuoha R. Relationship between transformational leadership and employee retention among healthcare professionals in the United States. Bus Econ Res. 2016;6(2):235–254. [Google Scholar]