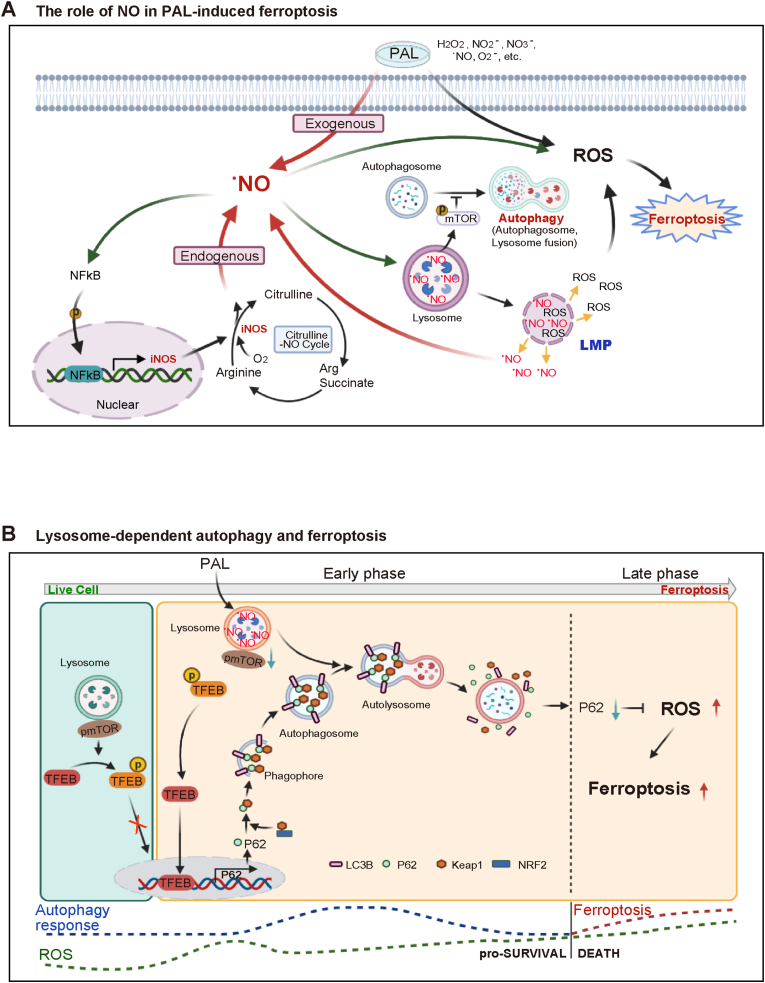
Fig. 6.
(A) Role of.NO in PAL-induced ferroptosis in MM cells. PAL provides MM cells with an immediate impact of exogenous . NO, a component of PAL. The initial stimulation of . NO activates transcription factor NF-κB in MM cells, upregulating the downstream iNOS as revealed by metabolome analysis. In return, this positive feedback loop leads to sustained accumulation of . NO in lysosomes. Simultaneously, .NO accumulation in lysosomes starts autophagic process, eventually resulting in lysosomal membrane permeabilization (LMP) upon certain threshold according with increase in lysosomal lipid peroxidation. The whole process eventually leads to ferroptosis. (B) Molecular switch from autophagy to ferroptosis in MM cells. In the absence of PAL stress, mTOR binds to p-TFEB, which keeps the autophagy event in a physiological level. Under PAL exposure, MM cells, by responding to exogenous reactive species, start autophagic processes, initialized by translocation of TFEB into the nucleus and the subsequent upregulation of p62 transcription. During this early event, autophagy serves as a survival mechanism and the following p62-Keap1–Nrf2 axis-mediated antioxidant response would keep the balance of ROS for a period of time. However, sustained accumulation of . NO-derived oxidants-mediated lipid peroxidation, and lysosomal dysfunction eventually results in ferroptosis, when autophagic process is terminated. The molecular switching of cell fate from autophagy for survival to ferroptosis occurs when autophagic proteins, such as p62, are downregulated as a consequence of accumulated lipid peroxidation in lysosomes.