| 1. Lipid nanoparticles |
-
•
Colloidal dispersions of solid particles with 0.1 up to 30% w/w of lipid(s), stabilized by one or two emulsifiers with a concentration range from 0.5% to 5% w/w;
-
•
Ability to encapsulate poor-water soluble drugs;
-
•
Industrial scale manufacture facility at low cost;
-
•
Good physical-chemical stability;
-
•
Improved drug bioavailability;
-
•
Ability for drug targeting;
-
•
Drug protection;
-
•
Prolonged drug release;
-
•
Use of generally recognised as safe (GRAS) excipients;
-
•
Low or no toxicity of formulations.
|
-
•
Allow the direct transport to the brain via trigeminal and olfactory nerves;
-
•
Improve bioavailability of drugs in the brain;
-
•
Ability for drug targeting;
-
•
Use of physiological lipids and GRAS excipients, providing high biocompatibility with the nasal mucosa and, therefore, low or no toxicity;
-
•
Ability to adhere to the olfactory epithelium;
-
•
Prolong the contact time of the formulation on the nasal mucosa, avoiding the fast mucociliary clearance;
-
•
Drug protection from nasal enzymatic degradation;
-
•
Prolonged drug release.
|
39, 40, 41, 42
|
1.1. Solid lipid nanoparticles (SLNs) 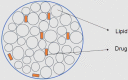
|
|
40, 43, 44
|
1.2. Nanostructured lipid carriers (NLCs) 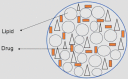
|
|
40, 45, 46
|
2. Nanoemulsions (NEs) 
|
-
•
Heterogeneous and thermodynamically unstable systems composed by an oily and a water phase, stabilized by emulsifier(s);
-
•
Oil-in-water (o/w) NE are more common and have ability to transport lipophilic molecules;
-
•
Droplet sizes lower than 500 nm;
-
•
Industrial scale production facility at low cost;
-
•
Good physical-chemical stability;
-
•
Improved drug bioavailability;
-
•
Ability for drug targeting;
-
•
Drug protection;
-
•
Prolonged drug release;
-
•
Use of physiological lipids and GRAS excipients;
-
•
Low or no toxicity of formulations.
|
47 |



