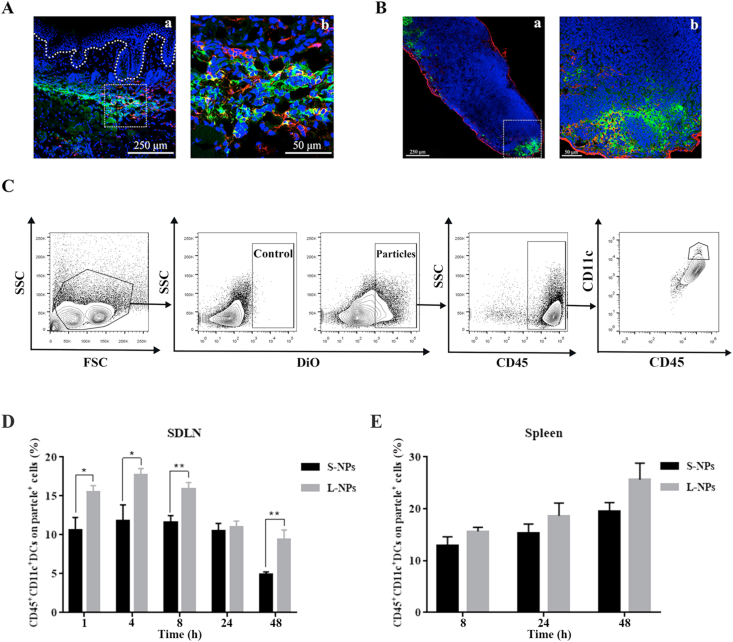
Figure 3.
DCs uptake preference of intradermal injected DiO-loaded PLGA nanoparticles in IMQ-induced psoriasis-like mice. (A) Representative immunofluorescence staining of psoriasis-like mice skin section (a) that intradermally injected with DiO-loaded PLGA nanoparticles (after injection for 4 h), enlarged in (b). (B) Representative immunofluorescence staining of SDLN from psoriasis-like mice that intradermally injected with DiO-loaded PLGA nanoparticles (after injection for 1 h), enlarged in (b). Scale bar: 250 μm (enlargement: 50 μm). Blue: nucleus, green: DiO, red: anti-CD11c. (C) Flow cytometry gating strategy to identify and analyze DCs populations in different lymphoid organs. Gating is shown in one representative data of mice at 1 h exposure after treated with S-NPs. After the initial live gating in a forward scatter (FSC) and side scatter (SSC) plot, particle-positive cells were first gated. Among them, DCs from SDLN and spleens were gated in a CD45 versus CD11c plot. (D) PBS controls and different sizes of DiO-loaded PLGA nanoparticles were intradermally administrated in IMQ-induced psoriasis-like mice, and the single-cell suspensions obtained from lymphoid organs were analyzed at 1, 4, 8, 24, and 48 h after particle exposure. Among particle-positive subset in SDLN (D) and spleens (E), comparison between S-NPs and L-NPs detected in these tissues were made. Data are presented as mean ± SEM (n = 6), ∗∗P < 0.01, ∗P < 0.05.