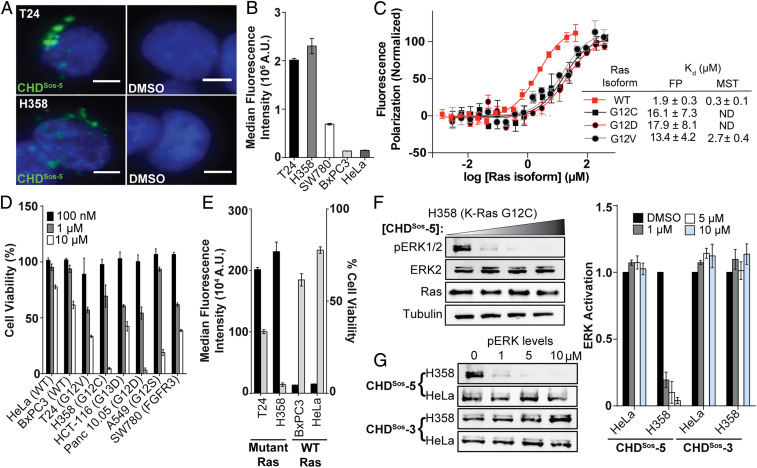
Fig. 4.
Cellular internalization and efficacy of Sos proteomimetics is modulated with oncogenic Ras mutations. (A) Live-cell fluorescence imaging of Hoechst-stained Ras mutant T24 and H358 cells incubated with fluorescently labeled CHDSos-5 or DMSO for 4 h at 40× magnification. (Scale bar, 5 μm.) (B) Flow cytometry analysis of fluorescently labeled CHDSos-5 (1 μM) in T24, H358, SW780, BxPC3, and HeLa cells after 1 h treatment. (C) FP and MST analyses were performed to determine the binding affinity of CHDSos-5 and GDP-loaded H-Ras wild-type and mutant isoforms. (D) The cellular toxicity of CHDSos-5 was analyzed in an MTT cell viability assay. Bar graph shows viability of Ras wild-type and mutant cell lines treated with increasing concentrations of CHDSos-5. The results from the MTT assay were confirmed in the CellTiter-Glo luminescent cell viability assay (SI Appendix). (E) Double y-axis graph shows correlation of CHDSos-5 cellular uptake and toxicity. Results from cellular uptake studies (left axis) with 1 μM fluorescent analog and MTT cell viability assay (right axis) at 10 μM concentration are shown. (F) Representative Western blot showing ERK phosphorylation levels in H358 cells upon treatment with 0, 1, 5, and 10 μM CHDSos-5. (G) Western blots showing ERK phosphorylation levels in HeLa and H358 cells upon treatment with CHDSos-5 or negative control CHDSos-3. Bar graphs compare ERK phosphorylation in HeLa and H358 cells posttreatment with CHDSos-3 and CHDSos-5. Error bars are mean ± SD of biological duplicates.