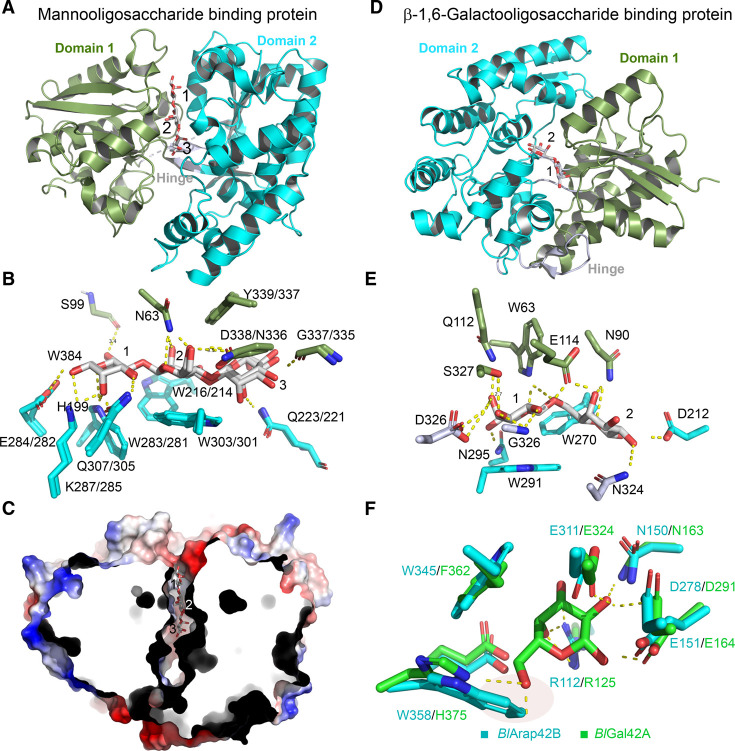
Figure 6. Transporter SBPs and enzymes of specialized utilization for plant-derived glycans by B. animalis subsp. lactis.
(A) Structure of the mannooligosaccharide binding protein from B. animalis subsp. lactis ATCC 27673 (BlMnBP1) in complex with mannotriose (PDB: 6I5V). (B) Differences in the binding site of the two homologous binding proteins BlMnBP1 and BlMnBP2 (PDB: 6FUV) that display high and low binding affinity to mannobiose, respectively. The amino acid residues are coloured according to the domain colour in (A). BlMnBP1/BlMnBP2 amino acid identities and numbering are shown. Asn63 is present only in BlMnBP1 and interacts with the mannosyl at subsite 2. (C) Cross-sectional view of the electrostatic surface of BlMnBP1 cocrystallised with mannopentaose (PDB: 6I5W) reveals additional space and cavities beyond subsite +3, consistent with the binding profile of larger or galactosyl-decorated oligosaccharides. (D) and (E) show the overall structure of the binding protein Bal6GBP (PDB: 6H0H) in complex with β1,6-galactoside and the detailed recognition of this ligand, respectively. (F) Comparison of the active site subsite –1 of the recently discovered α-l-arabinopyranosidase BlArap42B (PDB: 5XB7) and the β-1,6/1,3-β-galactosidase BlGal42A (PDB: 4UNI) in complex with galactose. A key difference is the substitution of a histidine that recognizes the C6-OH with a hydrogen bond in BlGal42A with a tryptophan residue that makes a steric clash with the C6-OH and instead is likely to stack onto the arabinopyranose that lacks this group.