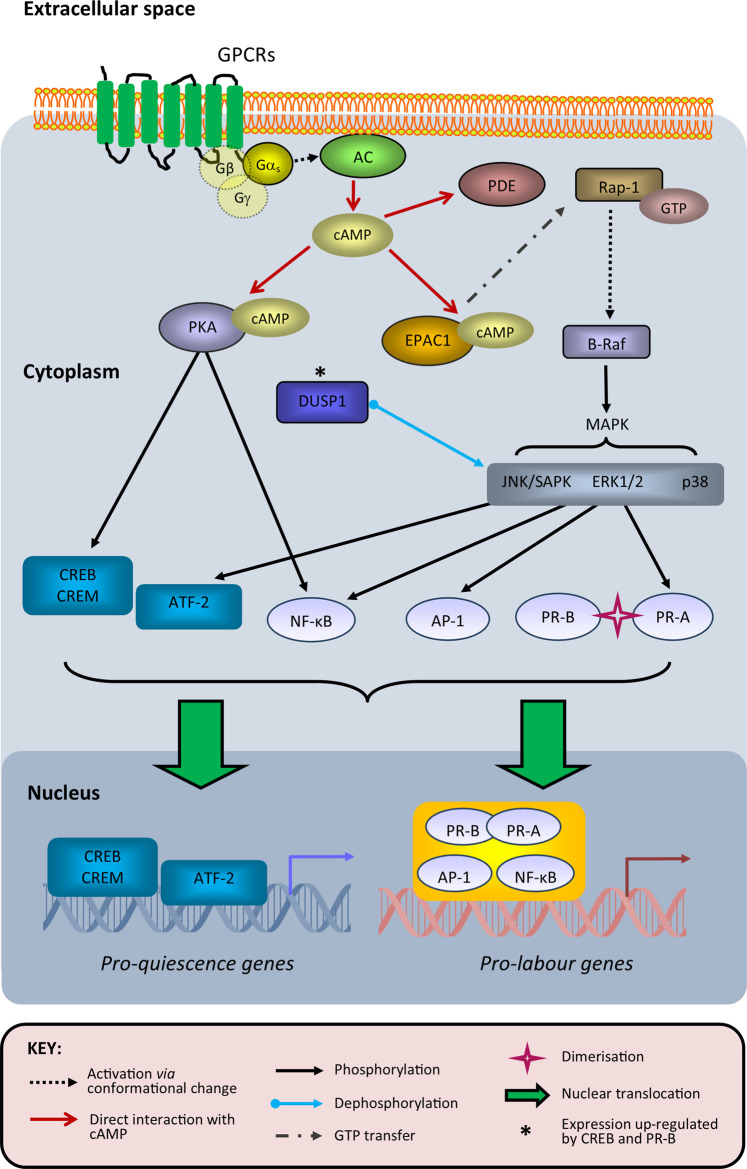
Figure 1. Overview of cAMP-regulated transcription factor activity in myometrial smooth muscle cells during pregnancy and labour.
Upon agonist binding to G-protein coupled receptors (GPCRs) that promote cyclic adenosine monophosphate (cAMP) signalling, Gαs dissociates from its trimeric G-protein complex to activate adenylate cyclase (AC) and thus increase cAMP synthesis. Elevation of cAMP concentrations increase the probability of cAMP to bind both regulatory subunits of each tetrameric protein kinase A (PKA) complex, which causes the dissociation and activation of both its catalytic subunits that subsequently phosphorylate proteins with exposed serine/threonine-containing motifs compatible to their active sites. These include transcription factors that bind to the cAMP response element (CRE) sequence within compatible gene promoters, such as CRE-binding protein (CREB) and CRE modulator (CREM); cAMP-dependent transcription factor 2 (ATF-2) can bind to CRE sites but is not a known PKA substrate. CREB and CREM activities have been proposed to promote expression of pro-quiescence genes, and this is potentially influenced by CREB heterodimerisation with ATF-2. PKA (via CREB) and PR-B activity can enhance expression of dual specificity phosphatase 1 (DUSP1), which dephosphorylates mitogen-activated protein kinases (MAPKs) to reduce phosphorylation of their downstream targets; these include progesterone receptor A (PR-A), which is phosphorylated at its Ser344/345 residue by c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) to promote its heterodimerisation and subsequent transrepression of PR-B. In addition to PKA, exchange protein directly activated by cAMP 1 (EPAC1) can also bind cAMP, which leads to activation of guanosine triphosphate (GTP)-bound Ras-like small GTPase 1 (Rap1); this promotes the phosphorylation activity of B-Raf to increase downstream MAPK signalling via extracellular signal-regulated kinase 1/2 (ERK1/2), JNK, SAPK and/or p38. Subsequently, activator protein 1 (AP-1) and nuclear factor κB (NF-κB) transcription factors enhance the expression of pro-labour genes, which can involve interaction with PR heterodimers. PKA and EPAC1 activities are reduced by phosphodiesterase (PDE)-mediated degradation of cAMP.