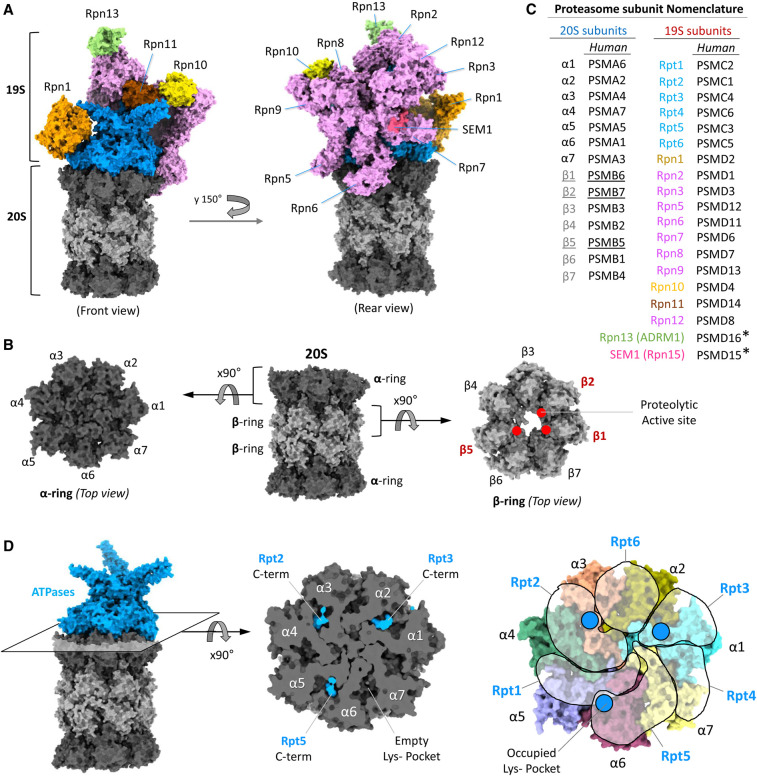
Figure 1. Subunit arrangement in the 26S proteasome.
(A) The 26S is made up of a 20S catalytic core (dark and light grey) and a 19S regulatory particle (coloured). Three ubiquitin receptors (Rpn1, orange; Rpn10, yellow, and Rpn13, green) are positioned peripherally on a ‘hood’ encompassing all other Rpn subunits (Rpn11 in brown, Sem1 in rose-pink and all the rest in pink) that partially surrounds a central ATPase motor (blue). (B) The side view of 20S-CP shows four rings stack above other. The top view of upper α-ring (with gate closed) and upper β-ring showing the seven homologous subunits in a resting proteasome. The red dots in the β-ring show the active sites of the three catalytic subunits. (C) Nomenclature of 26S proteasome subunits. Subunit colour coding corresponds to that of in panel (A). *PSMD15 has been approved by HGNC/HUGO as an alias for SEM1. PSMD16 has been approved by HGNC/HUGO as an alias for ADRM1. (D) ATPase ring interaction with α-ring of 20S CP (left panel). The cross section (middle panel) at the interaction interface shows the three C-terminal tails (HbYX motif; blue) of Rpt2/3/5 inserted into the corresponding three Lys-pockets (out of seven) on α-ring. Asymmetric arrangement of six ATPases on the ring of seven α-subunits (right panel). Each transparent free-shape represents an AAA-domain of each ATPase subunit. The blue circles indicate the position of C-terminal insertion into the Lys-pocket. The figures (in panel (A), (B) and (D)) are generated by ChimeraX using the model structure of resting 26S proteasome (PDB: 6j2x).