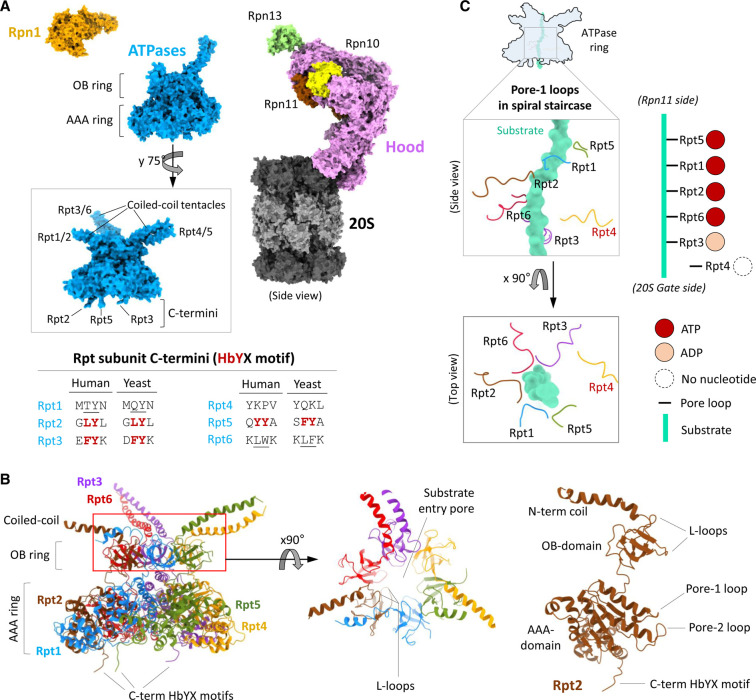
Figure 2. Structural details of the ATPase subunits of the 26S proteasome.
(A) Structurally, 19S RP subunits organise into a hood (pink, brown and yellow), an engine (blue; all ATPases) and a tetraUb holder (orange; Rpn1). The insert images of ATPases ring shows the coiled-coil tentacle pairs and the three extended C-terminus. The inserted table shows C-terminal residues of Rpt2/3/5 subunits with the conserved HbYX motif in human and Yeast. The underlined C-terminal residues in Rpt1/6 are pseudo-HbYX motif. (B) The cartoon model of the ATPases ring shows the structural features (left). The top view of OB ring (middle) shows the central pore for substrate entry and the six pairs of L-loops. The cartoon model of Rpt2 (right) shows the detailed structural features. The figures (in panel (A) and (B)) are generated by ChimeraX using the model structure of resting 26S proteasome (PDB: 6j2x). (C) The model figure represents a snapshot of the pore-1 loops of all six ATPases (Rpt) arranged in a spiral staircase surrounding a substrate polypeptide (in Aquamarine colour) inside the ATPase channel during the hand-over-hand catalytic cycle. In the staircase Rpt4 pore-1 loop is disengaged from the substrate. The figure is generated by ChimeraX using the model structure of substrate bound 26S proteasome (PDB: 6msk).