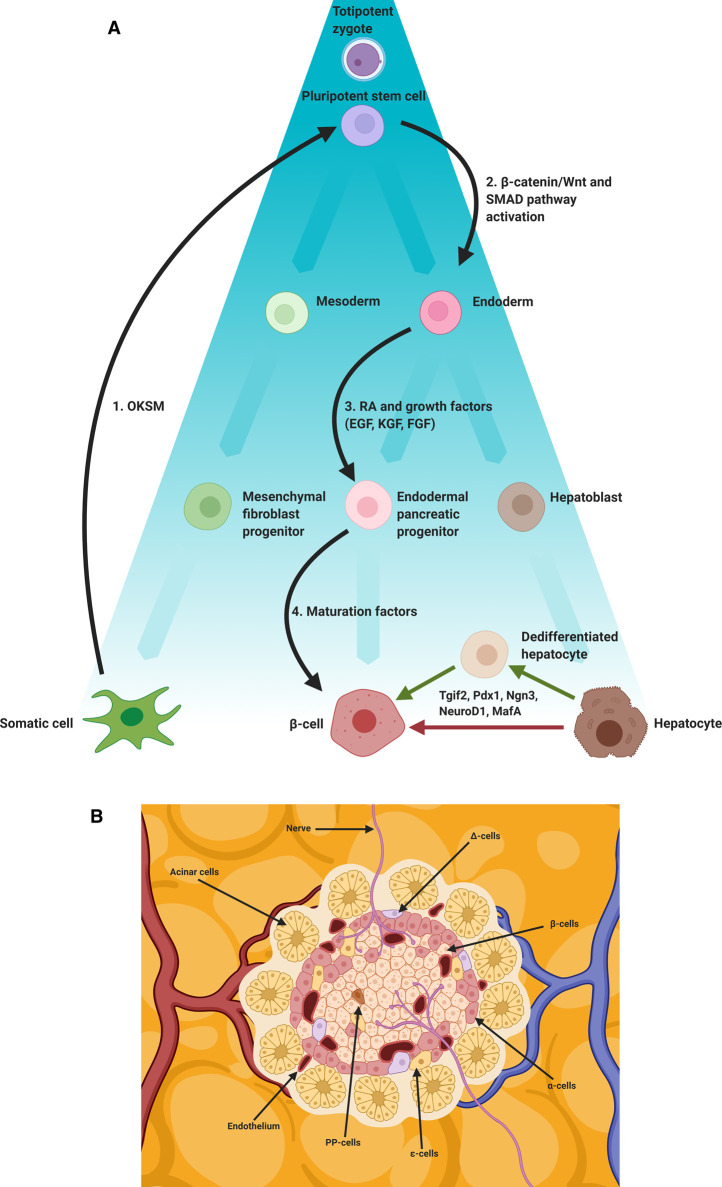
Figure 1. β-cell programming and reprogramming.
(A) Schematic representation of embryological differentiation from zygote to differentiated somatic cell (β-cell, hepatocyte), the directed differentiation of OKSM iPSCs to β-cell and the transdifferentiation of hepatocytes to β-cell using pancreatic master regulator transcription factors (Tgif2, Pdx1, Ngn3, NeuroD1, MafA). Black arrows indicate steps involved in the generation and subsequent programming of iPSCs; (1) Somatic cells transfected with OKSM transcription factors are reprogrammed to a pluripotent stem cell state using Yamanaka factors (OKSM), (2) the resulting iPSC can then be treated with small molecules, cytokines, morphogens and growth factors. Fate is directed toward an endoderm lineage, typically through activation of the β-catenin/Wnt and SMAD signalling pathways (3). Pancreatic progenitors are then induced, typically using retinoic acid (RA) in combination with a range of growth factors (EGF, KGF, FGF). Finally, (4) a β-cell phenotype is induced using various maturation factors. A recent study used a cocktail including Anaplastic Lymphoma Kinase Inhibitor II (ALKi), Triiodo-L-Thyronine (T3), PI3-K Inhibitor (XXI), ALK inhibitor LDN193189 (LDN) and vitamin C (Vc). Ectopic expression of pancreatic transcription factors (Pdx1, MafA, Ngn3, Tgif2, NeuroD1) can induce or contribute to transdifferentiation to a β-cell phenotype. Direct (no intermediary stage) and indirect (a dedifferentiated intermediary stage) routes are depicted. (B) β-cells reside in the islets of Langerhans and are subject to a specific niche involving mechanical and chemical cues. Blood borne factors (including oxygen (O2)), extra cellular matrix (ECM) components, neighbouring acinar, endocrine (α-cells, δ-cells, PP-cells, ε-cells), endothelial and neuronal cells all contribute to this niche.