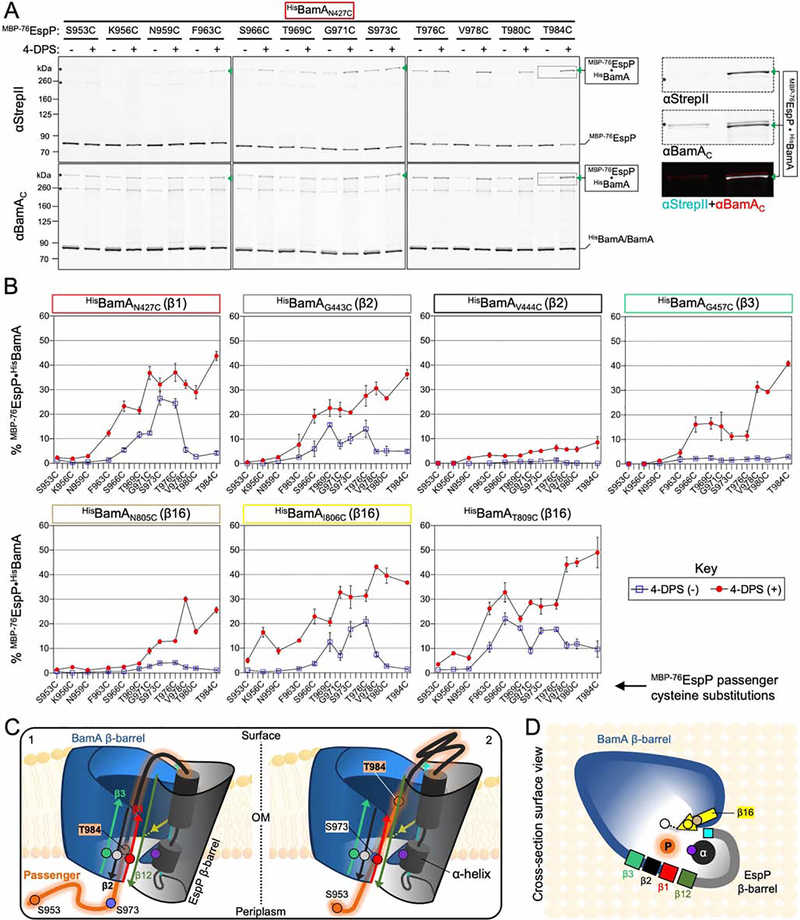
Figure 2: The autotransporter passenger secretion channel is located within the BamA lumen.
(A) E. coli BL21(DE3) expressing HisBamAN427CBCDE and MBP−76EspP with cysteine substitutions at the indicated residues were mock treated (−) or treated with 4-DPS (+). Intermolecular disulfide-bonds (•) between cysteine pairs were detected by double-immunoblotting with antibodies against the N-terminus of MBP−76EspP (αStrepII, top) or the C-terminus of BamA (αBamAC, bottom). Right, example of detected HisBamAN427C•MBP−76EspPT984C magnified from boxed region of immunoblot (and αStrepII (cyan) and αBamAC (red) signals overlaid). Asterisk: non-specific side-reactions. (B) Disulfide-bond levels (mean ± SEM) between MBP−76EspP and HisBamA for all cysteine substitutions defined in Figure 1. Experiments (N=3) conducted as in A except that immunoblots were probed with αStrepII only. Statistical tests are in Table S1. (C) Model of the autotransporter secretion channel based on disulfide-mapping. ‘1’ and ‘2’ portray the dynamicity of the passenger (orange) as it traverses the OM through the luminal space within the BamA-EspP hybrid-barrel. Some passenger residue positions used in A and B are depicted (orange/blue circles). EspP α-helix residue N1026 (purple circle) is also shown. BamA β1 (red), β2 (black), and β3 (green) are shown with luminal residues N427 (red circle), G443 (gray circle), and G457 (green circle), respectively. MBP prevents MBP−76EspP residues close to S953 from entry into the channel. (D) Surface-view cross-section of C. BamA β16 residues N805 (tan circle), I806 (yellow circle), and T809 (white circle), and EspP β1 (cyan square) are shown.