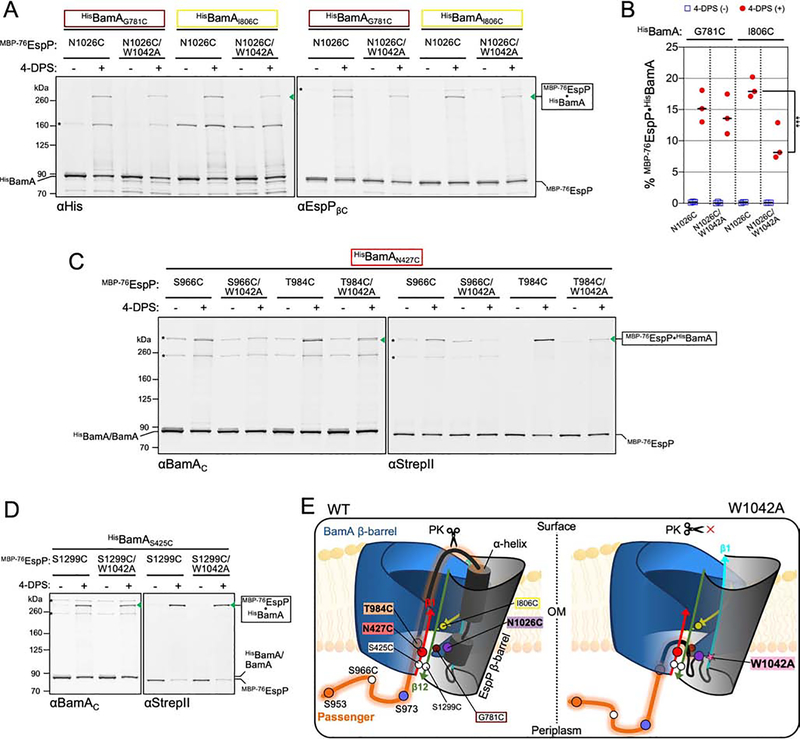
Figure 5: The conserved tryptophan is required to position the autotransporter passenger inside the BamA β-barrel.
(A) Experiments as in Figure 2A except strains expressed either MBP−76EspPN1026C or MBP−76EspPN1026C/W1042A (cysteine positioned in the α-helix) and either HisBamAG781CBCDE or HisBamAI806CBCDE and disulfide-bonded proteins were detected using αHis (left) and αEspPβC (right). Asterisk: non-specific side-reactions. (B) Experiments conducted as in A except immunoblots probed with αStrepII and disulfide-bonded MBP−76EspP⦁HisBamA was quantified (line at median, N = 3). Statistical tests are in Table S1, P < 0.001 (***). (C) As in A except that strains expressed HisBamAN427CBCDE and MBP−76EspP passenger cysteine substitutions (S966C or T984C) with or without the W1042A substitution. Immunoblots were doubly probed with αBamAC (left) and αStrepII (right) (N = 2). (D) As in C except that strains expressed MBP−76EspPS1299C (with or without the W1042A substitution) and HisBamAS425C (cysteines are in close proximity during hybrid-barrel state). (E) Model showing the importance of the conserved β1 tryptophan in passenger secretion. W1042A disrupts the positioning/folding of the α-helix into the BamA-EspP hybrid-barrel channel (and, in turn, the positioning of the passenger) such that no detectible translocation occurs. Labelling as in Figure 2C.