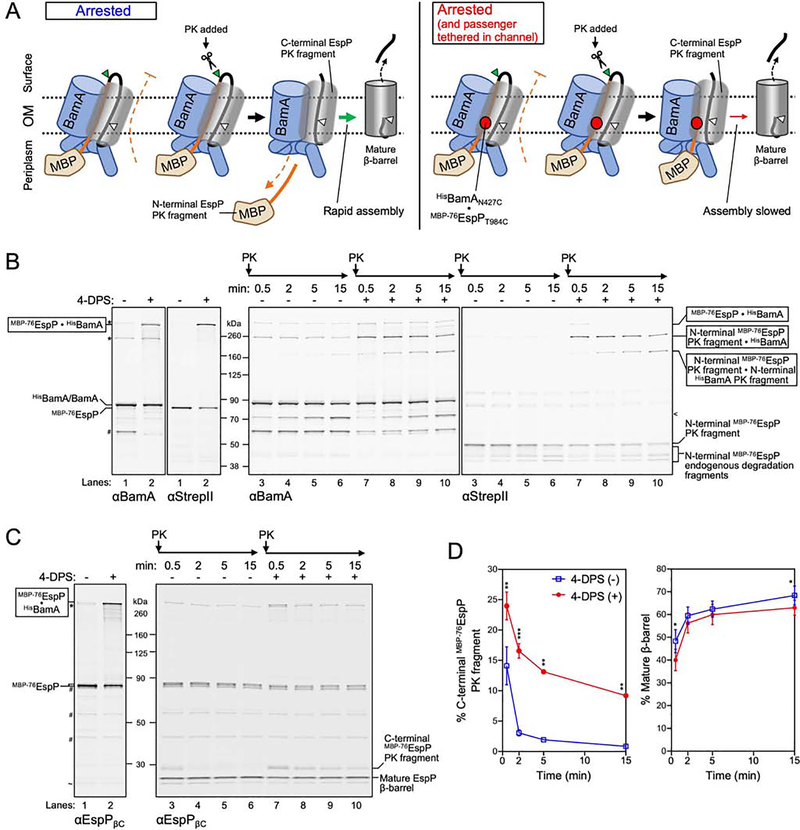
Figure 6: Channel occupancy by a translocating passenger inhibits completion of β-barrel assembly.
(A) Summary of experimental design and results in B-D. Left, the assembly of MBP−76EspP is arrested due to inhibition of passenger translocation by MBP. Assembly can be restarted by addition of PK, which digests the exposed passenger loop and allows rapid secretion of the C-terminus of the passenger and autocatalytic release of a mature β-barrel. Right, the passenger was additionally tethered to the secretion channel via a disulfide-bond. PK treatment resulted in a pool of C-terminal EspP fragments and slowed conversion into a mature β-barrel. (B, C) E. coli BL21(DE3) expressing HisBamAN427CBCDE and MBP−76EspPT984C were treated as in Figure 2A and subsequently treated with PK for up to 15 min. Disulfide-bonded species, fragment formation, and EspP β-barrel maturation, were detected by double-immunoblot using antisera against full length BamA (αBamA, left) and StrepII (right) (B), or using only αEspPβC (C). Side-reactions (*), non-specific bands (~), endogenous BamA degradation fragments (#), and BamA PK fragments (<) are denoted. (D) Quantified levels (mean ± SEM) of C-terminal EspP PK fragments (right) or mature β-barrel (far right) from experiments in C (N = 3). Statistical tests are in Table S1, P <0.05 (*), P < 0.01 (**), P < 0.001 (***). See also Figure S6.