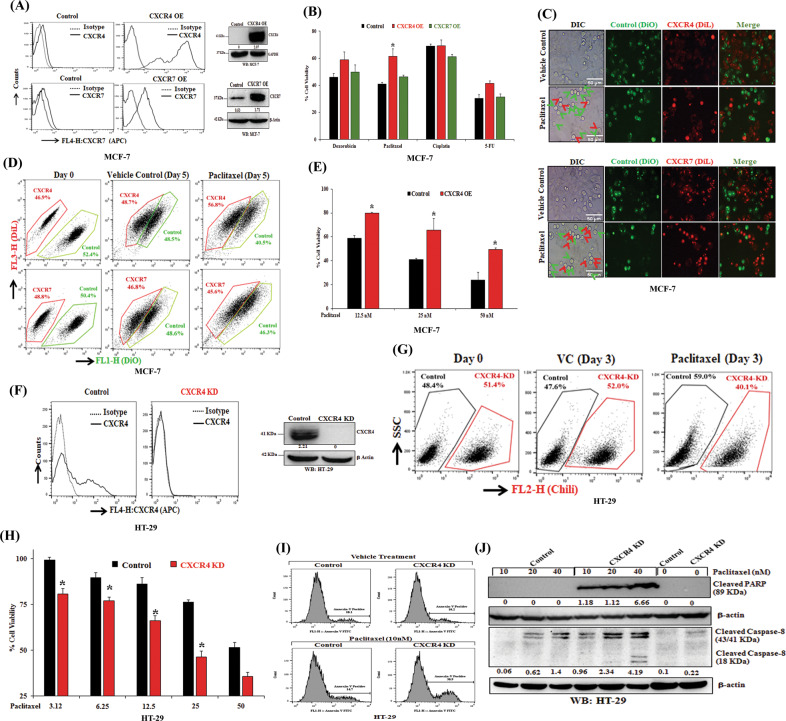
Fig. 1. CXCR4 regulates paclitaxel resistance in cancer.
A MCF7 cells were transfected with either empty vector pcDNA3.1 or vector containing the gene for the overexpression of either chemokine receptor CXCR4 or CXCR7 and made stable. These stable cell lines were stained with either APC-conjugated anti-human CXCR4 (CD184) or anti-human CXCR7 antibodies along with their respective isotype control antibodies and analyzed by flow cytometry. The surface expression levels of CXCR4 and CXCR7 are represented in histogram overlays (left panel). Western blot analysis of CXCR4 and CXCR7 in the lysate of control and CXCR4/CXCR7 overexpression MCF-7 cells; GAPDH and β-Actin were used as the protein loading control (right panel). B CXCR4 or CXCR7 overexpressing and control MCF7 cells were treated with Doxorubicin (250 nmol/L), Paclitaxel (25 nmol/L), Cisplatin (2.5 μmol/L), or 5-Fluorouracil (25 μmol/L) for 72 h and cytotoxicity was measured by SRB assay as described in materials and methods. Percent cell viability was tabulated. Columns, an average of triplicate readings of samples; error bars ± SEM. *p < 0.05, compared with control cells. C DiL (red) stained CXCR4 or CXCR7 overexpressing MCF7 cells and DiO (green) stained control MCF7 cells were mixed in equal numbers, seeded in 6-well plate and treated with vehicle or Paclitaxel (10 nmol/L) for 3 days and then analyzed via fluorescence microscopy; red arrows indicate DiL stained dead CXCR4 or CXCR7 overexpressing cells, while green arrows indicate DiO stained dead control cells. Photomicrographs are representative of three independent experiments. D DiL stained CXCR4 overexpressing or CXCR7 overexpressing MCF7 cells were equally mixed with DiO stained control MCF7 cells, and a small aliquot of mixture was acquired as day 0 reading by FACS. The rest of the cells were treated either with vehicle or Paclitaxel (10 nmol/L) for 5 days and subsequently analyzed by FACS. Data are representative of at least three independent experiments. E CXCR4 overexpressing and control MCF7 cells were treated with different concentrations of paclitaxel (12.5, 25, 50 nM) for 48 h, and cytotoxicity was evaluated by SRB assay. Percent cell viability was tabulated. Columns, an average of triplicate readings of samples; error bars ± SEM.*p < 0.05, compared to control cells treated with respective doses of paclitaxel. F Chili tagged HT-29 cells were made stable for the knockdown of CXCR4 via shRNA mediated lentiviral transduction; scramble shRNA transduced stable HT-29 cells were used as control. CXCR4 knockdown and control HT-29 cells were stained either with APC-conjugated anti-human CXCR4 (CD184) antibody or with appropriate isotype control antibody and analyzed by FACS. Histogram overlays represent the cell surface expression of CXCR4 (left panel). Western blot analysis of CXCR4 in the lysate of control and CXCR4 knockdown HT29 cells; β-Actin was used as the protein loading control (right panel). G Control and CXCR4 knockdown Chili tagged HT29 cells were mixed equally and analyzed by FACS either at day 0 or after three days of vehicle/paclitaxel (20 nmol/L) treatments. H CXCR4 knockdown and control HT-29 cells were treated with different concentrations of Paclitaxel (3.12, 6.25, 12.5, 25, 50 nM) for 48 h and cytotoxicity was measured by SRB assay. Percent cell viability was tabulated. Columns, an average of triplicate readings of samples; error bars ± SEM.*p < 0.05 compared to control cells. I Control and CXCR4 knockdown HT29 cells were treated with paclitaxel for 24 h and stained with FITC conjugated Annexin-V. Histogram overlays show the Annexin-V positive cells. J Western blot analysis of cleaved PARP and Caspase-8 in the lysate of 24 h post vehicle or Paclitaxel treated (10, 20, and 40 nM) control and CXCR4 knockdown HT-29 cells; β-Actin was used as the protein loading control. Western Blot densitometric quantification numbers are shown above the loading control blot of all immunoblot studies.