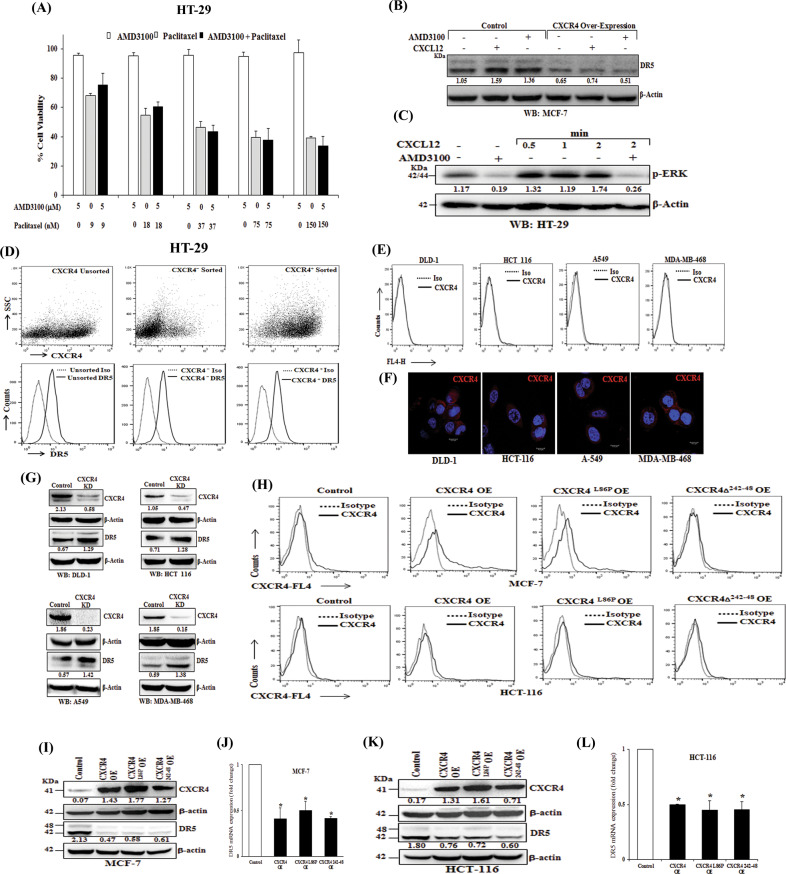
Fig. 4. CXCR4 mediated DR5 regulation is independent of CXCR4-CXCL12 signaling.
A HT-29 cells were treated with either different concentrations of paclitaxel (9, 18, 37, 75, 150 nM) or AMD3100 (5 μM) alone or in combinations for 48 h and cytotoxicity was measured by SRB assay. Percent cell viability was tabulated. Columns, an average of triplicate readings of samples; error bars ± SEM. B Control and CXCR4 overexpressed MCF-7 cells were treated with CXCR4 ligand CXCL12 (100 ng/ml) or CXCR4 antagonist AMD3100 (5 μmol/L) for 12 h, and subjected to Western blot analysis for DR5 and β-actin. C HT-29 cells were either pre-treated with vehicle or AMD3100 (5 μM) for 12 h, followed by treatment with CXCR4 ligand CXCL12 (100 ng/ml) for different time points (0.5, 1, and 2 mins) and subjected to Western blot analysis for p-ERK and β-actin. D CXCR4+ and CXCR4− HT-29 cells were flow-sorted and plated. After 5 days of culture, cells were stained with either APC-conjugated CXCR4 (CD184) and PE-conjugated DR5 or their respective matched isotype control antibodies and analyzed by FACS. In the upper panel, dot plots represent CXCR4 staining in unsorted, CXCR4+sorted and, CXCR4− sorted cells. In the lower panel histograms represent DR5 staining in the above-mentioned respective cells. E DLD-1, HCT-116, A-549, and MDA-MB-468 cells were stained with either APC-conjugated anti-human CXCR4 (CD184) or isotype control antibodies and analyzed by FACS. The cell surface expression of CXCR4 is represented in histogram overlays. F DLD-1, HCT-116, A-549, and MDA-MB-468 cells were seeded on coverslips for 24 h and subjected to immunofluorescence staining for CXCR4 and analyzed by confocal microscopy; Scale bar 10 μm. G DLD-1, HCT-116, A-549, and MDA-MB-468 cells were made stable for CXCR4 knockdown via shRNA mediated lentiviral transduction and scramble shRNA transduced cells were used as control. Immunoblot analysis of CXCR4 and DR5 protein in control or CXCR4 knockdown cells are shown; β-Actin was used as an internal protein loading control. H–L MCF-7 and HCT-116 cells were transfected with scrambled, wild type CXCR4, CXCR4L86P, or CXCR4δ242-248 containing vectors and cultured. After 48 h, cells were either stained with APC-conjugated anti-human CXCR4 (CD184)/isotype control antibodies and analyzed by FACS, or subjected to western blot or total RNA isolation. H The cell surface expression of CXCR4 is represented in histogram overlays. I, K Immunoblot analysis of CXCR4 and DR5 protein in control, wild type CXCR4, CXCR4L86P, or CXCR4δ242-248 transfected MCF-7 and HCT-116 cells; β-Actin was used as an internal protein loading control. Western Blot densitometric quantification numbers are shown above the loading control blot of all immunoblot studies. J, L Fold change in DR5 mRNA expression was measured by RT-qPCR as described in Materials and Methods. Data are representative of three independent experiments, resulting from duplicate readings of two different samples; Columns, average value of DR5 mRNA expression; bars ± SEM. *, p < 0.05, compared with respective controls.