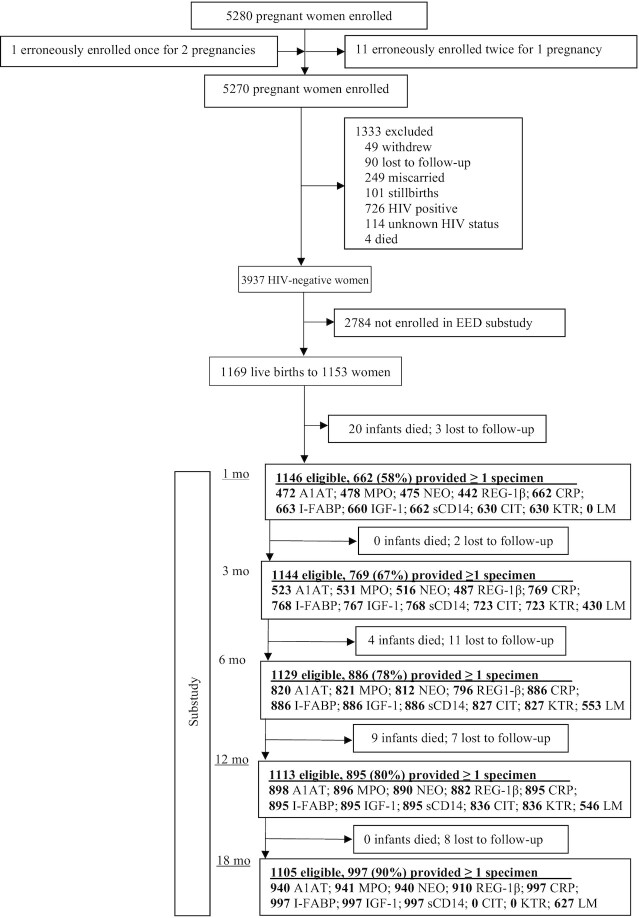
FIGURE 1.
Flow of participants through the trial. A1AT, fecal α-1 antitrypsin; CIT, plasma citrulline; CRP, plasma C-reactive protein; EED, environmental enteric dysfunction; I-FABP, plasma intestinal fatty acid binding protein; IGF-1, insulin-like growth factor 1; KTR, kynurenine-to-tryptophan ratio; LM, lactulose–mannitol; LMR, lactulose-to-mannitol ratio (urinary); MPO, fecal myeloperoxidase; NEO, fecal neopterin; REG-1β, fecal regenerating gene 1β; sCD14, plasma soluble CD14.